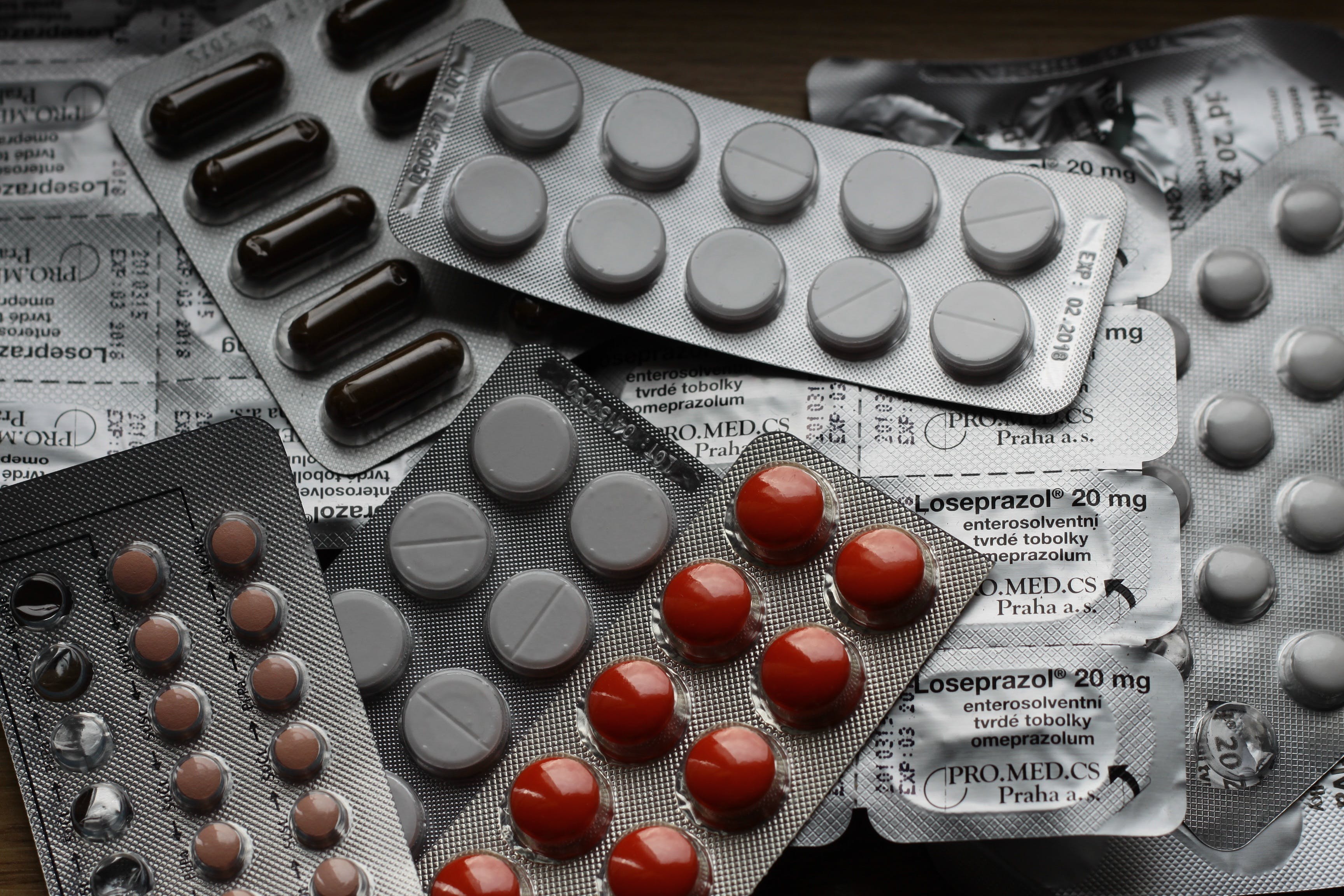
| Time limit as per Public Service Guarantee Act 21 days |
940
| Sr.No. | Circle | Applications Received | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | ADC Office, Solan (BBN) |
338 ADC Office, Solan (BBN)
Applications Received: 338
|
||||||
| 2 | ADC Office, Solan |
151 ADC Office, Solan
Applications Received: 151
|
||||||
| 3 | ADC Office, Kangra |
281 ADC Office, Kangra
Applications Received: 281
|
||||||
| 4 | ADC Office, Chamba |
95 ADC Office, Chamba
Applications Received: 95
|
||||||
| 5 | ADC Office, Bilaspur |
94 ADC Office, Bilaspur
Applications Received: 94
|
||||||
| 6 | ADC Office, Hamirpur |
129 ADC Office, Hamirpur
Applications Received: 129
|
||||||
| 7 | ADC Office, Kullu |
101 ADC Office, Kullu
Applications Received: 101
|
||||||
| 8 | ADC Office, Una |
144 ADC Office, Una
Applications Received: 144
|
||||||
| 9 | ADC Office, Mandi |
251 ADC Office, Mandi
Applications Received: 251
|
||||||
| 10 | ADC Office, Sirmaur |
136 ADC Office, Sirmaur
Applications Received: 136
|
||||||
| 11 | ADC Office, Kinnaur |
9 ADC Office, Kinnaur
Applications Received: 9
|
||||||
| 12 | ADC Office, Shimla |
195 ADC Office, Shimla
Applications Received: 195
|
696
106
680
₹ 2685050.37
| Sr.No. | Application No | Application Date | Approval Date | Fee Detail | Total Fee Charged |
|---|---|---|---|---|---|
| 1 | 65365 | 06/11/2024 | 15/10/2024 | RETAIL DRUGS LICENSE | 3000 |
| 2 | 65367 | 06/11/2024 | 08/10/2024 | GRANT OF RETAIL DRUG LICENCE | 3000 |
| 3 | 65369 | 06/11/2024 | 07/11/2024 | Fee for retail licence | 3000 |
| 4 | 65374 | 06/11/2024 | 26/03/2025 | Form 20 & 21 Both (Retail & Wholeshale) | 6000 |
| 5 | 65375 | 06/11/2024 | 08/10/2024 | A24I172974 & CPAEFCOBF0 | 3000 |
| 6 | 65376 | 06/11/2024 | 08/10/2024 | A24I295882 & 2112340168 | 3000 |
| 7 | 65381 | 06/11/2024 | 13/11/2024 | Fees For Retail Drug License | 3000 |
| 8 | 65382 | 06/11/2024 | 14/11/2024 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED ONLINE VIDE HIMGRN NO A24I257753 DATED 24-09-2024 | 3000 |
| 9 | 65383 | 06/11/2024 | 17/10/2024 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 10 | 65384 | 06/11/2024 | 15/10/2024 | A24I299889 & 21114739903 | 3000 |
| 11 | 65385 | 06/11/2024 | 30/10/2024 | Fee for grant of wholesale drug license | 3000 |
| 12 | 65388 | 06/11/2024 | 13/11/2024 | Fees For Retail Drug License | 3000 |
| 13 | 65390 | 06/11/2024 | 10/10/2024 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 14 | 65394 | 06/11/2024 | 29/10/2024 | Fee for grant of wholesale drug license | 3000 |
| 15 | 65392 | 06/11/2024 | 05/11/2024 | Cash | 3000 |
| 16 | 65389 | 06/11/2024 | 04/11/2024 | UPI Transection ID 464370963126 | 3000 |
| 17 | 65399 | 06/11/2024 | 15/10/2024 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 18 | 65396 | 06/11/2024 | 08/10/2024 | Fees for fresh retail drugs license | 3000 |
| 19 | 65404 | 06/11/2024 | 29/10/2024 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 20 | 65407 | 06/11/2024 | 15/10/2024 | HIMGRN.A24I267149 on Dated 25.09.2024 | 3000 |
| 21 | 65402 | 06/11/2024 | 23/10/2024 | FEE FOR GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 22 | 65409 | 06/11/2024 | 05/11/2024 | Chief Medical Officer Kalpa | 3000 |
| 23 | 65372 | 06/11/2024 | 21/01/2025 | grant | 3000 |
| 24 | 65414 | 06/11/2024 | 10/12/2024 | grant of license | 3000 |
| 25 | 65412 | 06/11/2024 | 23/10/2024 | B24I113695 at UCO Bank Ghumarwin on dated 19-09-2024 | 3000 |
| 26 | 65370 | 06/11/2024 | 03/12/2024 | grant of retail drugs license | 3000 |
| 27 | 65419 | 06/11/2024 | 05/11/2024 | For grant of drug retail licence | 3000 |
| 28 | 65424 | 06/11/2024 | 13/11/2024 | Payment of Rs 3000 vide HIMGRN NO. A24I258910 Dated 24/09/2024 | 3000 |
| 29 | 65425 | 06/11/2024 | 11/12/2024 | HIMGRN NO. B24I114340 DATED ON 19.09.2024 | 3000 |
| 30 | 65427 | 06/11/2024 | 04/11/2024 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 31 | 65428 | 06/11/2024 | 17/10/2024 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 32 | 65429 | 06/11/2024 | 23/10/2024 | Gaurav Sharma Shastri Medical Hall Dharampur, treasury SOL00/DDO 505 | 6000 |
| 33 | 65431 | 06/11/2024 | 20/11/2024 | Fees For Retail Drug License | 3000 |
| 34 | 65437 | 06/11/2024 | 17/10/2024 | HIMGRN.A24J133607 on Dated 04.10.2024 (Fee for Grant of Retail Sale Drugs License). | 3000 |
| 35 | 65438 | 06/11/2024 | 06/12/2024 | A24J223422 & 21262367536 | 3000 |
| 36 | 65405 | 06/11/2024 | 27/11/2024 | Fee for grant of RETAIL drug license | 3000 |
| 37 | 65441 | 06/11/2024 | 19/11/2024 | HIMGRN NO. A24I237445 DATED ON 20.09.2024 | 3000 |
| 38 | 65442 | 06/11/2024 | 04/11/2024 | A24J227043 & 21263996335 | 3000 |
| 39 | 65444 | 06/11/2024 | 28/10/2024 | Deposited through E chalan Net banking no. Refenece no. 212326667773 | 3000 |
| 40 | 65443 | 06/11/2024 | 05/11/2024 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 41 | 65421 | 06/11/2024 | 19/11/2024 | Fee for grant of RETAIL drug license | 3000 |
| 42 | 65456 | 06/11/2024 | 05/11/2024 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 43 | 65453 | 06/11/2024 | 23/10/2024 | BANK REFERENCE NO 21292782974, HIMGRN A24J259448, FEES FOR APPLICATION FOR GRANT OF RETAIL SALE DRUG LICENCES | 3000 |
| 44 | 65458 | 06/11/2024 | 19/11/2024 | Fees For Retail Drug License | 3000 |
| 45 | 65457 | 06/11/2024 | 21/01/2025 | RANA MEDICAL STORE -APPLICATION FEE FOR GRANT OF RETAIL DRUGS | 3000 |
| 46 | 65460 | 06/11/2024 | 02/12/2024 | Fees for retail & wholesale drugs licenses | 6000 |
| 47 | 65461 | 06/11/2024 | 04/12/2024 | GRANT OF RETAIL DRUG LICNESE | 3000 |
| 48 | 65461 | 06/11/2024 | 04/12/2024 | APPLICATION FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 49 | 65454 | 06/11/2024 | 26/10/2024 | FEE FOR GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 50 | 65466 | 06/11/2024 | 17/01/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 51 | 65468 | 06/11/2024 | 26/10/2024 | Fee for grant of RETAIL drug license | 3000 |
| 52 | 65475 | 06/11/2024 | 03/12/2024 | FOR RETAIL DRUG | 3000 |
| 53 | 65478 | 06/11/2024 | 25/11/2024 | Fee for grant of RETAIL drug license | 3000 |
| 54 | 65480 | 06/11/2024 | 18/12/2024 | Fee for wholesale drugs license | 3000 |
| 55 | 65473 | 06/11/2024 | 02/01/2025 | For wholesale drug license | 3000 |
| 56 | 65482 | 06/11/2024 | 05/02/2025 | Fees for the wholesale drugs license | 3000 |
| 57 | 65486 | 06/11/2024 | 04/12/2024 | Fee for grant of RETAIL drug license | 3000 |
| 58 | 65485 | 06/11/2024 | 28/11/2024 | FOR RETAIL DRUG | 3000 |
| 59 | 65472 | 06/11/2024 | 02/12/2024 | Fees for the retail drugs license | 3000 |
| 60 | 65492 | 06/11/2024 | 10/12/2024 | FORM-20 RETAIL | 3000 |
| 61 | 65449 | 06/11/2024 | 20/11/2024 | Fees For Retail Drug License | 3000 |
| 62 | 65496 | 06/11/2024 | 11/12/2024 | FORM-20 , 21 RETAIL | 3000 |
| 63 | 65450 | 06/11/2024 | 19/11/2024 | Fee for the Retail Drugs licence | 3000 |
| 64 | 65499 | 06/11/2024 | 10/12/2024 | Fees For Retail Drug License | 3000 |
| 65 | 65498 | 06/11/2024 | 27/11/2024 | Bank Ref no. CPAEIVLZ19, Himgrn no. A24J234412 | 3000 |
| 66 | 65500 | 06/11/2024 | 05/11/2024 | RADHIKA MEDICAL STORE | 3000 |
| 67 | 65502 | 06/11/2024 | 12/11/2024 | A24J320543 & 21352543160 | 3000 |
| 68 | 65497 | 06/11/2024 | 02/12/2024 | Fees for the retail drugs license | 3000 |
| 69 | 65504 | 06/11/2024 | 27/11/2024 | Bank code 21331635161, Him Grn no. A24J293122 | 6000 |
| 70 | 65505 | 06/11/2024 | 06/11/2024 | Fresh Retail Drug License Fee | 3000 |
| 71 | 65509 | 06/11/2024 | 14/11/2024 | Fees for the grant of wholesale drugs license | 3000 |
| 72 | 65519 | 06/11/2024 | 12/11/2024 | A24J343566 & 21371236398 | 3000 |
| 73 | 65521 | 06/11/2024 | 14/11/2024 | Fee for grant of wholesale drug license | 3000 |
| 74 | 65514 | 06/11/2024 | 21/01/2025 | Form 20 retail | 3000 |
| 75 | 65515 | 06/11/2024 | 02/12/2024 | Fees for the retail drugs license | 3000 |
| 76 | 65524 | 06/11/2024 | 10/12/2024 | Fees for the Grant of retail drugs license | 3000 |
| 77 | 65527 | 06/11/2024 | 04/11/2024 | B24I118173 on dated 24-09-2024 | 6000 |
| 78 | 65528 | 06/11/2024 | 14/11/2024 | Fees for the grant of Retail drugs license | 3000 |
| 79 | 65534 | 06/11/2024 | 12/11/2024 | A24J360476 & 21383478362 | 3000 |
| 80 | 65533 | 06/11/2024 | 05/11/2024 | FEES FOR GRANT OF RETAIL DRUGS LICENSE Rs 3000 SUBMITTED ONLINE VIDE HIMGRN NO A24J361090 DATED 26-10-2024 | 3000 |
| 81 | 65535 | 06/11/2024 | 05/11/2024 | FEES FOR GRANT OF RETAIL DRUGS LICENSE Rs 3000 SUBMITTED ONLINE VIDE HIMGRN NO A24J362644 DATED 26-10-2024 | 3000 |
| 82 | 65538 | 06/11/2024 | 18/12/2024 | Sales drug licenses fee | 6000 |
| 83 | 65537 | 06/11/2024 | 30/10/2024 | Fees for fresh retail drugs license | 3000 |
| 84 | 65542 | 06/11/2024 | 02/01/2025 | Fee for retail drugs license | 3000 |
| 85 | 65543 | 06/11/2024 | 08/11/2024 | B24J114640 on dated 22-10-2024 | 3000 |
| 86 | 65545 | 06/11/2024 | 04/11/2024 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 87 | 65544 | 06/11/2024 | 13/11/2024 | Fees for the retail drugs license | 3000 |
| 88 | 65541 | 06/11/2024 | 02/12/2024 | grant of retail drug lic. | 3000 |
| 89 | 65548 | 06/11/2024 | 27/11/2024 | Payment done on 18/10/2024 | 3000 |
| 90 | 65550 | 06/11/2024 | 27/11/2024 | Fee for grant of RETAIL drug license | 3000 |
| 91 | 65551 | 06/11/2024 | 06/11/2024 | Challan for the grant of Retail drugs license. | 3000 |
| 92 | 65386 | 06/11/2024 | 04/11/2024 | Sale Drug Licences-wholesale | 3000 |
| 93 | 65549 | 06/11/2024 | 08/11/2024 | B24J105766 on dated 08-10-2024 | 3000 |
| 94 | 65553 | 06/11/2024 | 14/11/2024 | Fee for grant of wholesale drug license | 3000 |
| 95 | 65554 | 06/11/2024 | 27/11/2024 | Fee for grant of RETAIL drug license | 3000 |
| 96 | 65556 | 06/11/2024 | 14/11/2024 | Fee for grant of wholesale drug license | 3000 |
| 97 | 65558 | 06/11/2024 | 27/11/2024 | Renewal fee | 3000 |
| 98 | 65522 | 06/11/2024 | 05/11/2024 | NEW REGISTRATION | 3000 |
| 99 | 65562 | 06/11/2024 | 12/11/2024 | CHAMUNDA MEDICAL STORE | 3000 |
| 100 | 65561 | 06/11/2024 | 20/11/2024 | PAID via cash at sbi una | 5998 |
| 101 | 65564 | 06/11/2024 | 25/11/2024 | Fee for grant of RETAIL drug license | 3000 |
| 102 | 65566 | 06/11/2024 | 29/11/2024 | Fees for the grant of Retail drugs license | 3000 |
| 103 | 65569 | 06/11/2024 | 17/01/2025 | Fee for grant of wholesale drug license | 3000 |
| 104 | 65572 | 06/11/2024 | 23/04/2025 | Fee for grant of RETAIL drug license | 3000 |
| 105 | 65575 | 07/11/2024 | 22/02/2025 | Wholesale Drug License Fee | 3000 |
| 106 | 65577 | 07/11/2024 | 11/12/2024 | FEE FOR THE RETAIL AND WHOLESALE DRUGS LICENSE | 6000 |
| 107 | 65536 | 08/11/2024 | 19/11/2024 | FEES FOR GRANT OF RETAIL DRUGS LICENSE Rs 3000 SUBMITTED ONLINE VIDE HIMGRN NO A24J363927 DATED 26-10-2024 | 3000 |
| 108 | 65587 | 08/11/2024 | 17/12/2024 | Fee for retail drugs license | 3000 |
| 109 | 65588 | 08/11/2024 | 10/12/2024 | grant of license | 3000 |
| 110 | 65585 | 11/11/2024 | 18/12/2024 | Fee for retail drugs license | 3000 |
| 111 | 65594 | 11/11/2024 | 03/01/2025 | grant of retail drug lic. | 3000 |
| 112 | 65601 | 12/11/2024 | 30/12/2024 | PAID THROUGH E CHALLAN | 3000 |
| 113 | 65578 | 12/11/2024 | 03/01/2025 | BOTH WHOLESALE & RETAIL | 6000 |
| 114 | 65605 | 13/11/2024 | 21/12/2024 | Retail Drug License Fee | 3000 |
| 115 | 65609 | 13/11/2024 | 27/11/2024 | Fee for the grant of Wholesale Drugs License. | 3000 |
| 116 | 65607 | 13/11/2024 | 18/12/2024 | Retail Drug License Fee | 3000 |
| 117 | 65613 | 13/11/2024 | 06/12/2024 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 118 | 65610 | 13/11/2024 | 02/01/2025 | Fees For Retail Drug License | 3000 |
| 119 | 65610 | 13/11/2024 | 02/01/2025 | Fees For Wholesale Drug License | 3000 |
| 120 | 65618 | 13/11/2024 | 22/04/2025 | 3000 | 3000 |
| 121 | 65616 | 13/11/2024 | 13/01/2025 | 3000 | 3000 |
| 122 | 65616 | 13/11/2024 | 13/01/2025 | 3000 | 3000 |
| 123 | 65565 | 14/11/2024 | 10/12/2024 | Fees for the retail drugs license | 3000 |
| 124 | 65526 | 14/11/2024 | 10/12/2024 | Fees for the wholesale drugs license | 3000 |
| 125 | 65603 | 14/11/2024 | 10/12/2024 | Fees for the retail drugs license | 3000 |
| 126 | 65623 | 14/11/2024 | 21/12/2024 | B24J293784 | 3000 |
| 127 | 65584 | 14/11/2024 | 01/02/2025 | grant of license | 3000 |
| 128 | 65620 | 14/11/2024 | 27/11/2024 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED IN UCO BANK ARKI VIDE HIMGRN NO B24K107343 DATED 12-11-2024 | 3000 |
| 129 | 65627 | 14/11/2024 | 06/01/2025 | Fee for wholesale drugs license | 3000 |
| 130 | 65441 | 16/11/2024 | 19/11/2024 | Firm has submitted fee for the change in constitution | 3000 |
| 131 | 65629 | 16/11/2024 | 18/12/2024 | FEE FOR GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 132 | 65631 | 16/11/2024 | 09/12/2024 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 133 | 65632 | 16/11/2024 | 11/12/2024 | 21372513612 ,21581620036 | 6000 |
| 134 | 65633 | 16/11/2024 | 29/11/2024 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 135 | 65634 | 16/11/2024 | 11/12/2024 | A24K147203 & 21491221982 | 3000 |
| 136 | 65636 | 16/11/2024 | 29/11/2024 | Fee for the Grant of Retail Drugs License. | 3000 |
| 137 | 65643 | 18/11/2024 | 09/12/2024 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 138 | 65645 | 18/11/2024 | 21/12/2024 | A24K249753 & 21599769749 | 3000 |
| 139 | 65640 | 19/11/2024 | 01/02/2025 | For wholesale drug license | 3000 |
| 140 | 65646 | 19/11/2024 | 18/12/2024 | Fee for retail drugs license | 3000 |
| 141 | 65647 | 19/11/2024 | 29/11/2024 | Apply for New Retail Drugs license | 3000 |
| 142 | 65651 | 19/11/2024 | 19/12/2024 | A24K256877 & 21608024426 | 3000 |
| 143 | 65659 | 21/11/2024 | 18/12/2024 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED IN UCO BANK ARKI VIDE HIMGRN NO A24K113524 DATED 21-11-2024 | 3000 |
| 144 | 65660 | 21/11/2024 | 09/12/2024 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 145 | 65662 | 21/11/2024 | 10/12/2024 | A24K275092 & 21626762045 | 3000 |
| 146 | 65664 | 22/11/2024 | 10/12/2024 | FEE FORWHOLESALE DRUG LICENSE | 3000 |
| 147 | 65665 | 22/11/2024 | 22/01/2025 | Fees for the grant of wholesale drugs license | 3000 |
| 148 | 65661 | 22/11/2024 | 19/12/2024 | A24K126702 | 3000 |
| 149 | 65663 | 22/11/2024 | 28/01/2025 | DDO 399 | 3000 |
| 150 | 65669 | 23/11/2024 | 18/12/2024 | FEES FOR GRANT OF RETAIL DRUGS LICENSE Rs 3000 SUBMITTED ONLINE VIDE HIMGRN NO A24K259291 DATED 19-11-2024 | 3000 |
| 151 | 65670 | 23/11/2024 | 06/12/2024 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 152 | 65626 | 23/11/2024 | 21/12/2024 | Fees for grant of retail of drug license | 3000 |
| 153 | 65672 | 25/11/2024 | 17/01/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 154 | 65674 | 26/11/2024 | 19/12/2024 | NAVEEN KUMAR SLAES DRUG LICENCES | 3000 |
| 155 | 65678 | 26/11/2024 | 11/12/2024 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 156 | 65680 | 27/11/2024 | 10/12/2024 | Fee for Grant of Retail Drugs License | 3000 |
| 157 | 65681 | 27/11/2024 | 15/01/2025 | A24K312755 & 21681440776 | 3000 |
| 158 | 65686 | 28/11/2024 | 21/12/2024 | Through UCO Bank Ghumarwin vide challan no. B24K113163 on dated 21-11-2024 | 6000 |
| 159 | 65687 | 28/11/2024 | 10/12/2024 | FRESH RETAIL LICENSE | 3000 |
| 160 | 65625 | 28/11/2024 | 18/12/2024 | HIMGRN: A24I123129 DATED 04/09/2024 BANK REF NO.- CPAEEIKGG9 | 3000 |
| 161 | 65688 | 28/11/2024 | 20/12/2024 | RETAIL DRUG LICENSE | 3000 |
| 162 | 65690 | 29/11/2024 | 02/04/2025 | DRUG LICENCE FEE | 3000 |
| 163 | 65692 | 29/11/2024 | 01/01/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 164 | 65695 | 30/11/2024 | 16/12/2024 | Deposited through e challan no. A24I273342 | 3000 |
| 165 | 65685 | 02/12/2024 | 06/12/2024 | NEW | 3000 |
| 166 | 65699 | 02/12/2024 | 21/12/2024 | GRAT OF RETAIL DRUG LICENSE | 3000 |
| 167 | 65530 | 03/12/2024 | 19/12/2024 | Grant of retail drug license | 3000 |
| 168 | 65704 | 03/12/2024 | 06/01/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 169 | 65703 | 04/12/2024 | 21/12/2024 | GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 170 | 65712 | 05/12/2024 | 19/12/2024 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 171 | 65711 | 05/12/2024 | 15/02/2025 | HIMGRN NO. A24L106956 DATED ON 02.12.2024 | 6000 |
| 172 | 65713 | 06/12/2024 | 11/12/2024 | RETAIL LICENSE | 3000 |
| 173 | 65718 | 06/12/2024 | 06/01/2025 | Fee for the Grant of Retail Drugs License. | 3000 |
| 174 | 65387 | 06/12/2024 | 24/01/2025 | Fees for fresh retail drugs license | 3000 |
| 175 | 65702 | 07/12/2024 | 19/12/2024 | B24K102273 dated 05-11-2024 | 3000 |
| 176 | 65725 | 09/12/2024 | 11/02/2025 | FEES GRANT OF WHOLE SALE DRUG LICENSES | 3000 |
| 177 | 65726 | 09/12/2024 | 06/01/2025 | RETAIL DRUG LICENSE | 3000 |
| 178 | 65728 | 10/12/2024 | 20/12/2024 | RETAIL DRUG LICENSE | 3000 |
| 179 | 65714 | 11/12/2024 | 06/01/2025 | GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 180 | 65590 | 11/12/2024 | 06/01/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 181 | 65721 | 11/12/2024 | 03/02/2025 | 0210-01-107-06(sale drug license) Fee for grant of retail drug license | 3000 |
| 182 | 65729 | 11/12/2024 | 24/01/2025 | ABHISHEK RANA (ABHINAV MEDICOS) | 3000 |
| 183 | 65693 | 13/12/2024 | 06/01/2025 | FEES FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 184 | 65737 | 13/12/2024 | 01/01/2025 | RETAIL DRUG LICENSE | 3000 |
| 185 | 65736 | 13/12/2024 | 13/01/2025 | SALES DRUG LICENCES -DRUG licence fee | 3000 |
| 186 | 65740 | 13/12/2024 | 21/12/2024 | FEE FOR GRANT OF RETAIL & WHOLESALE DRUG LICENSE | 6000 |
| 187 | 65742 | 16/12/2024 | 03/02/2025 | Fee for retail drugs license | 3000 |
| 188 | 65744 | 16/12/2024 | 03/02/2025 | Fees for grant of retail of drug license | 3000 |
| 189 | 65745 | 16/12/2024 | 21/01/2025 | Fee for retail drugs license | 3000 |
| 190 | 65739 | 16/12/2024 | 21/12/2024 | FEE FOR CHANGE IN PREMISES OF WHOLESALE DRUG LICENSE | 3000 |
| 191 | 65746 | 16/12/2024 | 03/01/2025 | HIMGRIN.A24L146893 on dated 07.12.2024 (Fee submitted for grant of Retail Sale Drugs License) | 3000 |
| 192 | 65715 | 16/12/2024 | 13/01/2025 | GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 193 | 65576 | 17/12/2024 | 30/12/2024 | HIMGRN NO. A24K142001 DATED ON 06.11.2024 | 3000 |
| 194 | 65751 | 17/12/2024 | 20/12/2024 | Fresh Retail Drug License Fees | 2997 |
| 195 | 65753 | 17/12/2024 | 10/01/2025 | Deposited Through E Challan No. A24L213181 dated 17.12.2024 | 3000 |
| 196 | 65593 | 18/12/2024 | 15/02/2025 | FOR RETAIL DRUG | 3000 |
| 197 | 65758 | 18/12/2024 | 14/05/2025 | HIMGRN NO. A24I261910 DATED ON 24.09.2024 | 3000 |
| 198 | 65759 | 18/12/2024 | 03/02/2025 | HIMGRN NO. A24L208865 DATED ON 17.12.2024 | 3000 |
| 199 | 65763 | 18/12/2024 | 03/02/2025 | FEE FOR THE RETAIL AND WHOLESALE DRUGS LICENSE | 6000 |
| 200 | 65762 | 18/12/2024 | 03/01/2025 | A24L221736 & 21889837964 | 3000 |
| 201 | 65761 | 19/12/2024 | 14/05/2025 | Cash payment of Three thousand rupees has been credited to the Government account under the head account 0210-01-107-06 at State bank of India, near old bus stand, Una by Khadwal medicos, Ward no 3, Rampur, Una (Proprietor: Nisha Rani) | 3000 |
| 202 | 65731 | 19/12/2024 | 13/01/2025 | GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 203 | 65766 | 19/12/2024 | 18/01/2025 | FOR GRANT RETAIL&WHOLESALE LICENSE | 6000 |
| 204 | 65768 | 19/12/2024 | 13/01/2025 | FOR GRANT OF RETAIL LICENCE | 3000 |
| 205 | 65769 | 19/12/2024 | 27/03/2025 | FOR GRANT OF RETAIL LICENSE | 3000 |
| 206 | 65772 | 20/12/2024 | 21/01/2025 | A24L237344 & 21907828016 | 3000 |
| 207 | 65774 | 20/12/2024 | 02/01/2025 | 0210-01-107-06(sale drug license) Fee for grant of retail drug license | 3000 |
| 208 | 65775 | 21/12/2024 | 09/01/2025 | RETAIL DRUG LICENSE | 3000 |
| 209 | 65776 | 21/12/2024 | 14/02/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 210 | 65716 | 21/12/2024 | 02/01/2025 | Fees for Grant retail & wholesale drugs licenses | 6000 |
| 211 | 65777 | 21/12/2024 | 06/05/2025 | GRANT RETAIL DRUG LICENSE | 3000 |
| 212 | 65778 | 21/12/2024 | 06/01/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 213 | 65730 | 21/12/2024 | 01/03/2025 | FOR GRANT OF WHOLESALE DRUGS LICENCES | 3000 |
| 214 | 65781 | 21/12/2024 | 09/01/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 215 | 65573 | 22/12/2024 | 01/02/2025 | Fees for grant of retail of drug license | 3000 |
| 216 | 65783 | 23/12/2024 | 01/01/2025 | Fresh Retail Drug License Fees | 3000 |
| 217 | 65784 | 23/12/2024 | 03/02/2025 | Fees for grant of retail of drug license | 3000 |
| 218 | 65770 | 23/12/2024 | 03/02/2025 | FOR RETAIL DRUG | 3000 |
| 219 | 65760 | 23/12/2024 | 03/02/2025 | FOR RETAIL DRUG | 3000 |
| 220 | 65786 | 23/12/2024 | 21/01/2025 | Fee for grant of Retailk & Wholesale drugs License | 6000 |
| 221 | 65787 | 23/12/2024 | 27/01/2025 | Fees for the grant of Retail drugs license | 3000 |
| 222 | 65789 | 23/12/2024 | 03/01/2025 | A24L153285 | 3000 |
| 223 | 65794 | 24/12/2024 | 22/01/2025 | RETAIL DRUG LICENSE | 3000 |
| 224 | 65795 | 24/12/2024 | 21/03/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 225 | 65799 | 26/12/2024 | 13/01/2025 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED ONLINE VIDE HIMGRN NO A24L118278 DATED 24-12-2024 | 3000 |
| 226 | 65800 | 26/12/2024 | 13/01/2025 | Fees for Grant of retail & wholesale drugs licenses | 6000 |
| 227 | 65764 | 26/12/2024 | 06/01/2025 | Fees for grant of retail & wholesale drugs licenses | 6000 |
| 228 | 65801 | 27/12/2024 | 21/01/2025 | Maa Mansa Medical Store | 3000 |
| 229 | 65803 | 28/12/2024 | 13/01/2025 | Fees For Grant of Retail Drug License | 3000 |
| 230 | 65805 | 30/12/2024 | 15/01/2025 | Through UCO Bank Ghumarwin vide challan no. B24K117054 on dated 26-11-2024 | 3000 |
| 231 | 65807 | 30/12/2024 | 13/01/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 232 | 65733 | 31/12/2024 | 15/01/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 233 | 65809 | 31/12/2024 | 09/01/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 234 | 65810 | 31/12/2024 | 09/01/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 235 | 65811 | 01/01/2025 | 03/02/2025 | fee for the grant of retail and wholesale drugs license | 6000 |
| 236 | 65812 | 01/01/2025 | 21/01/2025 | HIMGRIN.A24L178400 on dated 12.12.2024 (Fee submitted for grant of Retail Sale Drugs License) | 3000 |
| 237 | 65813 | 01/01/2025 | 15/01/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 238 | 65815 | 02/01/2025 | 28/01/2025 | Fee for the Grant of Retail Drugs License. | 3000 |
| 239 | 65820 | 02/01/2025 | 10/01/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 240 | 65823 | 02/01/2025 | 27/01/2025 | 0210-01-107-06 (SALES DRUG LICENSE) | 3000 |
| 241 | 65822 | 03/01/2025 | 14/02/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 242 | 65826 | 03/01/2025 | 03/02/2025 | retail drugs license | 3000 |
| 243 | 65827 | 03/01/2025 | 15/01/2025 | Through UCO Bank Ghumarwin vide challan no.B24L115567 on dated 20-12-2024 | 6000 |
| 244 | 65829 | 03/01/2025 | 13/01/2025 | Wholesale Drug License Fee | 2998 |
| 245 | 65831 | 03/01/2025 | 06/01/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 246 | 65830 | 03/01/2025 | 07/03/2025 | Fee for wholesale drugs license | 3000 |
| 247 | 65838 | 04/01/2025 | 23/01/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 248 | 65842 | 06/01/2025 | 23/01/2025 | Fees for the grant of Retail drugs license | 3000 |
| 249 | 65848 | 07/01/2025 | 20/06/2025 | GRANT WHOLESALE DRUG LICENSE | 3000 |
| 250 | 65849 | 07/01/2025 | 15/02/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 251 | 65855 | 07/01/2025 | 17/01/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 252 | 65854 | 07/01/2025 | 15/01/2025 | 10000 | 1000 |
| 253 | 65837 | 08/01/2025 | 28/03/2025 | Fees for the retail and wholesale drugs license | 6000 |
| 254 | 65861 | 08/01/2025 | 05/02/2025 | Fee for the grant of Wholesale Drugs License. | 3000 |
| 255 | 65862 | 08/01/2025 | 03/02/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 256 | 65864 | 08/01/2025 | 22/01/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 257 | 65865 | 08/01/2025 | 17/01/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 258 | 65867 | 08/01/2025 | 23/01/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 259 | 65866 | 09/01/2025 | 22/01/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 260 | 65870 | 09/01/2025 | 03/02/2025 | HIMGRN NO. B24L107681 DATED ON 10.12.2024 | 3000 |
| 261 | 65873 | 09/01/2025 | 15/02/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 262 | 65880 | 10/01/2025 | 21/03/2025 | Fees for grant of retail of drug license | 3000 |
| 263 | 65879 | 10/01/2025 | 23/01/2025 | payment for new retail drug license | 3000 |
| 264 | 65818 | 10/01/2025 | 05/02/2025 | FEE FOR GRANT OF WHOLESALE DRUGS LICENSE | 3000 |
| 265 | 65878 | 10/01/2025 | 25/02/2025 | Fees for grant of retail of drug license | 3000 |
| 266 | 65883 | 10/01/2025 | 24/01/2025 | A25A197008 & 22116273811 | 3000 |
| 267 | 65875 | 10/01/2025 | 03/02/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3120 |
| 268 | 65860 | 13/01/2025 | 21/01/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 269 | 65888 | 14/01/2025 | 11/02/2025 | Deposited Through E Challan No. A25A172986 dated 08.01.2025 | 3000 |
| 270 | 65886 | 14/01/2025 | 11/02/2025 | Deposited Through E-challan no. A25A137693 dated 04.01.2025 | 3000 |
| 271 | 65890 | 14/01/2025 | 31/01/2025 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED ONLINE VIDE HIMGRN NO A24L148851 DATED 08-12-2024 | 3000 |
| 272 | 65891 | 14/01/2025 | 19/02/2025 | Deposited through e-challan no. A25A216898 dated 14.01.2025 | 3000 |
| 273 | 65881 | 15/01/2025 | 24/01/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 274 | 65892 | 15/01/2025 | 11/02/2025 | HIMGRIN.A24G26799 and A24G267643 on dated 24.07.2024 (Fee submitted for grant of Retail Sale and Wholesale Drugs License) | 6000 |
| 275 | 65882 | 15/01/2025 | 21/01/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 276 | 65900 | 16/01/2025 | 15/02/2025 | Deposited Through Echallan no. A25A226469 dated 15.01.2025 | 3000 |
| 277 | 65903 | 17/01/2025 | 03/02/2025 | Retail Drug License Fee | 3000 |
| 278 | 65856 | 17/01/2025 | 11/02/2025 | HIMGRIN.A25A140407 on dated 04.01.2025 (Fee submitted for grant of Retail Sale and Wholesale Drugs License) | 6000 |
| 279 | 65898 | 17/01/2025 | 12/03/2025 | Fee for Grant of retail drug license | 3000 |
| 280 | 65904 | 17/01/2025 | 29/01/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 281 | 65909 | 18/01/2025 | 14/02/2025 | Grant of retail drug license | 3000 |
| 282 | 65910 | 18/01/2025 | 24/01/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 283 | 65911 | 18/01/2025 | 24/01/2025 | FEE FOR GRANT OF WHOLE SALE DRUG LICENSE | 3000 |
| 284 | 65915 | 20/01/2025 | 05/02/2025 | Fee for the Grant of Retail Drugs License. | 3000 |
| 285 | 65906 | 20/01/2025 | 29/01/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 286 | 65917 | 20/01/2025 | 24/01/2025 | Fees for fresh retail drugs license | 3000 |
| 287 | 65918 | 20/01/2025 | 15/02/2025 | Fresh Retail Drug License Fees | 2998 |
| 288 | 65921 | 20/01/2025 | 18/02/2025 | Fees for the retail drugs license | 3000 |
| 289 | 65923 | 20/01/2025 | 15/02/2025 | REATIL & WHOLESALE | 6000 |
| 290 | 65929 | 21/01/2025 | 12/03/2025 | Fees for the retail drugs license | 3000 |
| 291 | 65932 | 21/01/2025 | 03/02/2025 | GRANT RETAIL DRUG LICENSE | 3000 |
| 292 | 65924 | 21/01/2025 | 05/02/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 293 | 65936 | 21/01/2025 | 05/02/2025 | RETAIL DRUG LICENSE | 3000 |
| 294 | 65937 | 21/01/2025 | 20/03/2025 | Fees for the retail drugs license | 3000 |
| 295 | 65938 | 21/01/2025 | 03/02/2025 | RETAIL DRUG LICENSE | 3000 |
| 296 | 65599 | 22/01/2025 | 21/03/2025 | Fees for grant of retail of drug license | 3000 |
| 297 | 65945 | 22/01/2025 | 07/03/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 298 | 65700 | 22/01/2025 | 10/02/2025 | Fees for grant of retail of drug license | 3000 |
| 299 | 65902 | 23/01/2025 | 10/02/2025 | Fees for grant of retail of drug license | 3000 |
| 300 | 65947 | 23/01/2025 | 21/03/2025 | Retail and Wholesale Drug License Fee | 6000 |
| 301 | 65885 | 23/01/2025 | 18/02/2025 | Applicarion for grant of retail drug license | 3000 |
| 302 | 65953 | 24/01/2025 | 14/02/2025 | A25A300957 & 22255204786 | 3000 |
| 303 | 65949 | 24/01/2025 | 03/02/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 304 | 65950 | 25/01/2025 | 03/02/2025 | DDO to chief medical officer kalpa | 3000 |
| 305 | 65954 | 27/01/2025 | 14/02/2025 | RETAIL LICENSE | 3000 |
| 306 | 65893 | 27/01/2025 | 18/02/2025 | Fee for Grant of retail drug license | 3000 |
| 307 | 65958 | 27/01/2025 | 27/06/2025 | FOR RETAIL DRUG | 3000 |
| 308 | 65959 | 27/01/2025 | 14/02/2025 | A25A297525 & 22253265608 | 3000 |
| 309 | 65961 | 27/01/2025 | 25/02/2025 | Fees for Grant of retail & wholesale drugs licenses | 6000 |
| 310 | 65948 | 27/01/2025 | 21/02/2025 | HIMGRIN.A25A284497on dated 22.01.2025 (Fee submitted for grant of Retail Sale Drugs License) | 6000 |
| 311 | 65963 | 28/01/2025 | 15/02/2025 | Fees for the grant of Retail and Wholesale drugs license | 6000 |
| 312 | 65968 | 28/01/2025 | 15/02/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 313 | 65556 | 28/01/2025 | 14/11/2024 | Fee for Change in Constitution (Change in firm name) | 3000 |
| 314 | 65969 | 28/01/2025 | 27/05/2025 | FOR RETAIL DRUG | 3000 |
| 315 | 65844 | 28/01/2025 | 23/04/2025 | Fee for wholesale drugs license | 3000 |
| 316 | 65970 | 28/01/2025 | 19/02/2025 | FEES FOR NEW WHOLESALES DRUG LICENCES | 3000 |
| 317 | 65946 | 29/01/2025 | 15/02/2025 | Wholesale Drug License Fee | 3000 |
| 318 | 65973 | 29/01/2025 | 14/02/2025 | B24L112561 on dated 17-12-2024 | 3000 |
| 319 | 65975 | 29/01/2025 | 05/05/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 320 | 65977 | 29/01/2025 | 29/03/2025 | RETAIL DRUG LICENSE | 3000 |
| 321 | 65979 | 30/01/2025 | 28/03/2025 | GRANT WHOLESALE DRUG LICENSE | 3000 |
| 322 | 65980 | 30/01/2025 | 05/02/2025 | HIMGRN NO. A25A195935 DATED ON 10.01.2025 | 3000 |
| 323 | 65982 | 30/01/2025 | 21/03/2025 | Fee for wholesale drugs license | 3000 |
| 324 | 65984 | 30/01/2025 | 24/02/2025 | RETAIL DRUG LICENSE | 3000 |
| 325 | 65547 | 30/01/2025 | 10/02/2025 | Fees for grant of retail of drug license | 3000 |
| 326 | 65985 | 30/01/2025 | 02/03/2025 | GRANT OF RETAIL AND WHOLESALE DRUG LICENSE | 6000 |
| 327 | 65992 | 30/01/2025 | 12/03/2025 | Fees FOR RETAIL DRUG | 3000 |
| 328 | 65993 | 31/01/2025 | 25/02/2025 | Fees for GRANT ofRetail drugs license | 3000 |
| 329 | 65994 | 31/01/2025 | 28/03/2025 | Fees for the retail drugs license | 3000 |
| 330 | 65978 | 01/02/2025 | 15/02/2025 | Fees for fresh wholesale drugs license. | 3000 |
| 331 | 66001 | 01/02/2025 | 25/02/2025 | Fees for the grant of retail drugs license | 3000 |
| 332 | 65996 | 01/02/2025 | 06/05/2025 | Fee for Retention of retail of drug license | 3000 |
| 333 | 65999 | 03/02/2025 | 02/03/2025 | Grant of Retail and Wholesale Drug License | 6000 |
| 334 | 65851 | 04/02/2025 | 07/03/2025 | GRANT OF WHOLESALE DRUGS LICENSE | 3000 |
| 335 | 66004 | 04/02/2025 | 03/03/2025 | HIMGRIN.A25A298163 on dated 24.01.2025 (Fee submitted for grant of Retail Sale Drugs License) | 2999 |
| 336 | 65943 | 04/02/2025 | 27/02/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 337 | 65788 | 04/02/2025 | 15/02/2025 | Fee for Grant of Retail & Wholesale drug license | 6000 |
| 338 | 66013 | 04/02/2025 | 05/02/2025 | Fee for Grant of Retail & Wholesale drug license | 6000 |
| 339 | 66015 | 05/02/2025 | 15/02/2025 | Fee for GRANT of wholesale drug license | 3000 |
| 340 | 65952 | 05/02/2025 | 19/02/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 341 | 66026 | 06/02/2025 | 12/03/2025 | Fee for the Grant of Retail Drugs License. | 3000 |
| 342 | 66038 | 06/02/2025 | 04/03/2025 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED THROUGH UCO BANK ARKI VIDE HIMGRN NO B25B104682 DATED 06-02-2025. | 3000 |
| 343 | 66049 | 07/02/2025 | 19/02/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 344 | 66057 | 10/02/2025 | 12/03/2025 | HIMGRN NO. A25B131559 DATED ON 05.02.2025 | 3000 |
| 345 | 66030 | 10/02/2025 | 10/03/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 346 | 66073 | 11/02/2025 | 19/02/2025 | Grant Of License | 6000 |
| 347 | 66076 | 11/02/2025 | 21/02/2025 | A25B172111 & CPAEUKHBQ5 | 3000 |
| 348 | 66071 | 13/02/2025 | 12/03/2025 | Fee for the Grant of Retail Drugs License. | 3000 |
| 349 | 66086 | 13/02/2025 | 04/03/2025 | Deposited through e challan no. A25B117418 | 3000 |
| 350 | 66098 | 14/02/2025 | 04/03/2025 | Deposited Through e challan no. A25B160908 | 3000 |
| 351 | 66099 | 14/02/2025 | 28/03/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 352 | 66100 | 14/02/2025 | 21/02/2025 | A25B196436 & CPAEUSSYI2 | 3000 |
| 353 | 66101 | 14/02/2025 | 01/04/2025 | Deposited through e challan no. A25A243476 | 3000 |
| 354 | 66105 | 15/02/2025 | 28/02/2025 | Fees For Wholesale Drug License | 3000 |
| 355 | 66106 | 15/02/2025 | 27/02/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 356 | 66012 | 15/02/2025 | 21/02/2025 | A25B124635 & 22372438399 | 3000 |
| 357 | 66108 | 15/02/2025 | 27/03/2025 | Sucessful | 3000 |
| 358 | 66112 | 17/02/2025 | 28/03/2025 | GRANT OF RETAIL DRUG LICENSE | 3000 |
| 359 | 66115 | 17/02/2025 | 02/04/2025 | FOR GRANT OF DRUG RETAIL LICENCE | 3000 |
| 360 | 66116 | 17/02/2025 | 02/04/2025 | Fees for the retail and wholesale drugs license | 6000 |
| 361 | 65747 | 17/02/2025 | 27/02/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 362 | 66122 | 17/02/2025 | 28/02/2025 | FEE FOR FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 363 | 66124 | 17/02/2025 | 28/02/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 364 | 66127 | 18/02/2025 | 04/03/2025 | fees for grant of retail drugs license | 3000 |
| 365 | 66009 | 18/02/2025 | 07/03/2025 | FEES FOR GRANT OF WHOLESALE DRUGS LICENSE 3000 SUBMITTED ONLINE VIDE HIMGRN NO A25B122814 DATED 04-02-2025 | 3000 |
| 366 | 66129 | 19/02/2025 | 02/03/2025 | A25A104260 on dated 01-01-2025 | 3000 |
| 367 | 66133 | 20/02/2025 | 28/03/2025 | Retail Drug License Fee | 3000 |
| 368 | 66134 | 20/02/2025 | 29/03/2025 | Fresh Retail Drug License Fee | 3000 |
| 369 | 66138 | 21/02/2025 | 10/03/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 370 | 66141 | 21/02/2025 | 10/03/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 371 | 66119 | 21/02/2025 | 07/03/2025 | E-CHALLAN FOR GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 372 | 66144 | 21/02/2025 | 12/03/2025 | Retail Drug License Fee | 3000 |
| 373 | 66148 | 24/02/2025 | 12/03/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 374 | 65366 | 25/02/2025 | 08/10/2024 | Duplicate License | 150 |
| 375 | 66125 | 25/02/2025 | 11/03/2025 | Deposited through e-challan no. A25B128866 dated 05.02.2025 | 3000 |
| 376 | 66150 | 25/02/2025 | 21/03/2025 | RETAIL DRUGS LICENSE | 3000 |
| 377 | 66147 | 25/02/2025 | 14/03/2025 | HIMGRIN.A25B244620 on dated 02.19.2025 (Fee submitted for grant of Retail Sale Drugs License) | 3000 |
| 378 | 66151 | 25/02/2025 | 29/03/2025 | HIMGRIN.A25B230873 on dated 18.02.2025 (Fee submitted for grant of Retail Sale Drugs License) | 3000 |
| 379 | 66146 | 25/02/2025 | 07/03/2025 | E-CHALLAN FOR GRANT OF WHOLESALE DRUGS LICENSE | 3000 |
| 380 | 65366 | 25/02/2025 | 08/10/2024 | GODOWN LICENSE | 3000 |
| 381 | 65556 | 27/02/2025 | 14/11/2024 | Fee for Change in Constitution & Change in firm Name. | 3000 |
| 382 | 66161 | 28/02/2025 | 29/03/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 383 | 65942 | 28/02/2025 | 10/03/2025 | Fees for the grant of Retail drugs license | 3000 |
| 384 | 66162 | 28/02/2025 | 02/04/2025 | A25B300710 & CPAEWDCXS7 | 3000 |
| 385 | 66163 | 28/02/2025 | 12/03/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 386 | 66164 | 28/02/2025 | 28/03/2025 | FEE FOR RETAIL DRUG LICENSE | 3000 |
| 387 | 66166 | 28/02/2025 | 28/03/2025 | FEE FOR RETAIL DRUG LICENSE | 3000 |
| 388 | 66157 | 01/03/2025 | 02/04/2025 | Grant of retail drug license | 3000 |
| 389 | 66152 | 01/03/2025 | 10/03/2025 | A25B189542 | 3000 |
| 390 | 66136 | 01/03/2025 | 13/03/2025 | E-Challan for Grant of Retail & Wholesale Drugs License | 6000 |
| 391 | 66174 | 03/03/2025 | 12/03/2025 | HIMGRN NO. A25B273758 DATED ON 24.02.2025 | 3000 |
| 392 | 65854 | 03/03/2025 | 15/01/2025 | Godown License | 3000 |
| 393 | 66165 | 03/03/2025 | 28/03/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 394 | 66173 | 03/03/2025 | 02/05/2025 | Fee for the Grant of Retail Drugs License. | 3000 |
| 395 | 66187 | 05/03/2025 | 14/03/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE. | 3000 |
| 396 | 66185 | 05/03/2025 | 21/03/2025 | Retail Drugs License | 3000 |
| 397 | 66191 | 06/03/2025 | 30/04/2025 | HIMGRN NO. A25B293384 DATED ON 27.02.2025 | 3000 |
| 398 | 66198 | 06/03/2025 | 24/04/2025 | HIMGRN NO. A25C123001 DATED ON 04.03.2025 | 3000 |
| 399 | 66208 | 06/03/2025 | 28/03/2025 | E-CHALLAN FOR GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 400 | 66209 | 06/03/2025 | 27/03/2025 | Payment Done | 3000 |
| 401 | 66210 | 07/03/2025 | 10/03/2025 | New License | 3000 |
| 402 | 66213 | 07/03/2025 | 29/03/2025 | Retail Drug License Fee | 3000 |
| 403 | 65814 | 07/03/2025 | 15/03/2025 | Fees for fresh retail drugs license | 3000 |
| 404 | 66222 | 07/03/2025 | 14/05/2025 | Fees for GRANT of retail and wholesale drug license | 6000 |
| 405 | 66224 | 10/03/2025 | 24/03/2025 | Retail Drug License Fee | 3000 |
| 406 | 66240 | 11/03/2025 | 27/03/2025 | Fees For Fresh Wholesale Drug License | 3000 |
| 407 | 66243 | 11/03/2025 | 04/04/2025 | A25C176667 TOTAL AM.3000 BANK REF. CPAEXEWHX9 | 3000 |
| 408 | 66249 | 11/03/2025 | 24/03/2025 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED VIDE HIMGRN NO B25C106956 DATED 11-03-2025 | 3000 |
| 409 | 66251 | 11/03/2025 | 24/03/2025 | GRAT OF RETAIL & WHOLESALE DRUG LICENSE | 6000 |
| 410 | 66252 | 11/03/2025 | 15/03/2025 | New License | 3000 |
| 411 | 66257 | 12/03/2025 | 27/05/2025 | Fee for wholesale drugs license | 3000 |
| 412 | 66256 | 12/03/2025 | 07/04/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 413 | 65773 | 12/03/2025 | 02/04/2025 | A24L239105 & 21909010565 | 3000 |
| 414 | 66262 | 12/03/2025 | 27/03/2025 | Fees For Fresh Retail Drug License | 3000 |
| 415 | 66269 | 13/03/2025 | 28/03/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 416 | 66274 | 13/03/2025 | 28/03/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 417 | 66255 | 13/03/2025 | 04/04/2025 | E-CHALLAN FOR GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 418 | 66275 | 13/03/2025 | 28/03/2025 | FEE FOR GRANT OF RETAIL & WHOLESALE DRUG LICENSE | 6000 |
| 419 | 66284 | 14/03/2025 | 15/05/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 420 | 66287 | 15/03/2025 | 28/03/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 421 | 66289 | 15/03/2025 | 01/04/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 422 | 66295 | 20/03/2025 | 21/03/2025 | Fee for fresh Retail & Wholesale Drugs License | 6000 |
| 423 | 66297 | 21/03/2025 | 27/03/2025 | A25C199246 & CPAEXLEHX4 (through Online payment) | 3000 |
| 424 | 66307 | 21/03/2025 | 04/04/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 425 | 66308 | 21/03/2025 | 05/05/2025 | GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 426 | 66310 | 21/03/2025 | 18/06/2025 | A25C267366 & CPAEYEUIL4 | 3000 |
| 427 | 66303 | 21/03/2025 | 29/03/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 428 | 66314 | 21/03/2025 | 02/04/2025 | A25C226630 & CPAEXSSJP6 | 3000 |
| 429 | 66315 | 21/03/2025 | 29/03/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 430 | 66312 | 21/03/2025 | 08/04/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 431 | 66318 | 22/03/2025 | 02/04/2025 | A25C267235 & CPAEYETKN7 Through in line payment | 3000 |
| 432 | 65374 | 22/03/2025 | 26/03/2025 | Retention | 3000 |
| 433 | 66321 | 22/03/2025 | 04/04/2025 | FEE FOR RETAIL DRUG LICENSE | 3000 |
| 434 | 66323 | 22/03/2025 | 06/05/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 435 | 66324 | 22/03/2025 | 29/03/2025 | FEE FOR GRANT OF RETAIL AND WHOLESALE DRUG LICENSE | 6000 |
| 436 | 66331 | 22/03/2025 | 28/04/2025 | Deposited through E-challan no. A25C235277 dated 18.03.2025 | 3000 |
| 437 | 66332 | 22/03/2025 | 04/04/2025 | Retail & Wholesale Drug License Fee | 6000 |
| 438 | 66319 | 22/03/2025 | 17/04/2025 | GRANT OF RETAIL DRUG LICENCE | 3000 |
| 439 | 66334 | 23/03/2025 | 30/04/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 440 | 66335 | 24/03/2025 | 19/05/2025 | Fee for the grant of Wholesale Drugs License. | 3000 |
| 441 | 65753 | 24/03/2025 | 10/01/2025 | FOR CHANGE AND ALTERATION OF PREMISES | 3000 |
| 442 | 66348 | 24/03/2025 | 29/03/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 443 | 66354 | 25/03/2025 | 09/04/2025 | Deposited through E-challan no. A25C123182 dated 04.03.2025 | 3000 |
| 444 | 66356 | 25/03/2025 | 24/04/2025 | HIMGRN NO. B25C106603 DATED ON 10.03.2025 | 3000 |
| 445 | 66346 | 25/03/2025 | 28/04/2025 | HIMGRN NO. B25C109492 DATED ON 13.03.2025 | 3000 |
| 446 | 66350 | 25/03/2025 | 04/06/2025 | FOR GRANT RETAIL&WHOLESALE LICENSE | 6000 |
| 447 | 66363 | 25/03/2025 | 28/04/2025 | Deposited Rs. 6000/- through e challan no. A25A342462 dated 30.01.2025 | 6000 |
| 448 | 66362 | 25/03/2025 | 07/04/2025 | Retail & Wholesale Drug License Fee | 6000 |
| 449 | 66365 | 25/03/2025 | 30/04/2025 | Fees for the retail drugs license | 3000 |
| 450 | 66338 | 25/03/2025 | 19/05/2025 | E-CHALLAN FOR GRANT OF WHOLESALE DRUGS LICENSE | 3000 |
| 451 | 66367 | 25/03/2025 | 26/03/2025 | Schedule X Retail | 3000 |
| 452 | 66368 | 27/03/2025 | 08/04/2025 | Retail & Wholesale Drug License Fee | 5995 |
| 453 | 66296 | 27/03/2025 | 07/04/2025 | E-challan | 3000 |
| 454 | 66371 | 27/03/2025 | 01/04/2025 | Deposited through e-challan no. A25B110962 dated 03.02.2025 | 3000 |
| 455 | 66375 | 28/03/2025 | 14/05/2025 | Sales Drug License payment | 3000 |
| 456 | 66379 | 28/03/2025 | 08/04/2025 | A25B301606 dated 28-02-2025 | 3000 |
| 457 | 66382 | 28/03/2025 | 08/04/2025 | Payment for sales Drug License | 3000 |
| 458 | 66387 | 28/03/2025 | 26/04/2025 | RETENTION OF RETAIL & WHOLESLAEDRUG LICENSE | 6000 |
| 459 | 66391 | 29/03/2025 | 28/04/2025 | Retail Drug License Fee | 3000 |
| 460 | 66396 | 29/03/2025 | 06/05/2025 | Fees for grant of retail of drug license | 3000 |
| 461 | 66402 | 01/04/2025 | 14/05/2025 | Fee for Grant of Retail Drugs License | 3000 |
| 462 | 66413 | 01/04/2025 | 09/04/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 463 | 66278 | 01/04/2025 | 11/04/2025 | GRANT OF RETAIL DRUG LICENSE | 3000 |
| 464 | 66419 | 02/04/2025 | 03/04/2025 | rETAIL LICENSE | 1500000 |
| 465 | 66421 | 02/04/2025 | 25/06/2025 | HIMGRN NO. B25C114773 DATED ON 19.03.2025 | 3000 |
| 466 | 66426 | 02/04/2025 | 06/05/2025 | HIMGRN NO. B25C116107 DATED ON 20.03.2025 | 3000 |
| 467 | 66429 | 02/04/2025 | 02/04/2025 | wholeale | 3000 |
| 468 | 66419 | 02/04/2025 | 03/04/2025 | Test | 30000 |
| 469 | 66432 | 02/04/2025 | 06/05/2025 | HIMGRN NO. A25C292931 DATED ON 24.03.2025 | 3000 |
| 470 | 66419 | 02/04/2025 | 03/04/2025 | REtension of wholesale license | 3000 |
| 471 | 66419 | 02/04/2025 | 03/04/2025 | 10000 | 1 |
| 472 | 66435 | 02/04/2025 | 03/04/2025 | test wholesale | 10000 |
| 473 | 66053 | 02/04/2025 | 18/04/2025 | sayma khatton house no 3, Roura Sector Bilaspur | 6000 |
| 474 | 66419 | 03/04/2025 | 03/04/2025 | TEst | 3000 |
| 475 | 66419 | 03/04/2025 | 03/04/2025 | Godown | 4000 |
| 476 | 66419 | 03/04/2025 | 03/04/2025 | Godown | 3000 |
| 477 | 66419 | 03/04/2025 | 03/04/2025 | Duplicate | 20324 |
| 478 | 66419 | 03/04/2025 | 03/04/2025 | CHANGE IN PREMISES | 12000 |
| 479 | 66419 | 04/04/2025 | 03/04/2025 | chnge in constituions | 1203 |
| 480 | 66454 | 04/04/2025 | 30/04/2025 | Deposited through e challan no. A25C140803 dated 06.03.2025 | 3000 |
| 481 | 66455 | 04/04/2025 | 15/05/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 482 | 66449 | 04/04/2025 | 18/04/2025 | A25C326960 on dated 27-03-2025 | 3000 |
| 483 | 66292 | 04/04/2025 | 17/04/2025 | FEE FOR GRANT OF RETAIL & WHOLESALE DRUG LICENSE | 6000 |
| 484 | 66451 | 04/04/2025 | 14/05/2025 | Grant of Wholesale Drug License | 3000 |
| 485 | 65833 | 04/04/2025 | 17/04/2025 | E-CHALLAN FOR GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 486 | 66388 | 04/04/2025 | 05/05/2025 | E-CHALLAN FOR GRANT OF RETAIL & WHOLESALE DRUGS LICENSE | 6000 |
| 487 | 66475 | 05/04/2025 | 21/04/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 488 | 66478 | 05/04/2025 | 17/04/2025 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED ONLINE VIDE HIMGRN NO A25D174537 DATED 05-04-2025 | 3000 |
| 489 | 66491 | 07/04/2025 | 12/06/2025 | A25D184589 & CPAEZUVFK3 | 3000 |
| 490 | 66493 | 07/04/2025 | 14/05/2025 | Fees for GRANT of retail drug license | 3000 |
| 491 | 66495 | 07/04/2025 | 06/05/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 492 | 66496 | 07/04/2025 | 18/06/2025 | A25D192091 & CPAEZVZZH8 | 3000 |
| 493 | 66456 | 07/04/2025 | 14/05/2025 | Joginder Singh Sunny medical store | 3000 |
| 494 | 66508 | 08/04/2025 | 17/04/2025 | FEE FOR GRANT OF RETAIL AND WHOLESALE DRUG LICENSE | 6000 |
| 495 | 66518 | 08/04/2025 | 30/04/2025 | Fee for Grant of Retail Drugs License | 3000 |
| 496 | 66474 | 08/04/2025 | 18/06/2025 | Fee for grant of Retail & Wholesale Drugs License. | 6000 |
| 497 | 66501 | 09/04/2025 | 30/04/2025 | HIMGRN No: A25D214502 Bank Ref. No: CPAFAAFZS2 | 3000 |
| 498 | 66533 | 09/04/2025 | 19/05/2025 | NETBANKING THRU SBI SEC 37 CHANDIGARH | 3000 |
| 499 | 66536 | 10/04/2025 | 03/05/2025 | Grant of Retail D.L | 3000 |
| 500 | 66537 | 10/04/2025 | 30/06/2025 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED in UCO BANK Vide HIMGRN NO B25D104739 DATED 05-04-2025 | 3000 |
| 501 | 66542 | 10/04/2025 | 24/04/2025 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED IN UCO BANK ARKI VIDE HIMGRN NO B25D103955 DATED 07-04-2025 | 3000 |
| 502 | 66549 | 10/04/2025 | 30/04/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 503 | 66554 | 11/04/2025 | 18/04/2025 | Fees for fresh retail drugs license | 3000 |
| 504 | 66558 | 11/04/2025 | 05/05/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 505 | 66559 | 11/04/2025 | 02/06/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 506 | 66563 | 11/04/2025 | 16/05/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENCES | 3000 |
| 507 | 66569 | 15/04/2025 | 30/04/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 508 | 66572 | 16/04/2025 | 25/06/2025 | Fee for GRANT of wholesale drug license | 3000 |
| 509 | 66574 | 16/04/2025 | 24/04/2025 | Fee for the Grant of Retail Drugs License. | 3000 |
| 510 | 66576 | 16/04/2025 | 24/04/2025 | E-CHALLAN | 3000 |
| 511 | 66309 | 16/04/2025 | 26/05/2025 | A25C269601MINOTI MAJUMDAR BANK REF. CPAEYFJJU6 | 3000 |
| 512 | 66584 | 16/04/2025 | 22/04/2025 | Fees For Retail Drug License | 3000 |
| 513 | 66586 | 17/04/2025 | 06/05/2025 | Fees for the retail drugs license | 3000 |
| 514 | 66588 | 17/04/2025 | 18/06/2025 | A25D290406 & CPAFAQOZF6 | 3000 |
| 515 | 66589 | 17/04/2025 | 28/04/2025 | New Retail drugs License Fee | 3000 |
| 516 | 66596 | 17/04/2025 | 24/04/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 517 | 66597 | 17/04/2025 | 22/04/2025 | Fees For Fresh Retail Drug License | 3000 |
| 518 | 66599 | 18/04/2025 | 27/05/2025 | BY UPI | 3000 |
| 519 | 65854 | 19/04/2025 | 15/01/2025 | For the Retention of Retail License | 3000 |
| 520 | 66598 | 21/04/2025 | 24/04/2025 | Fees For FRESH Retail Drug License | 3000 |
| 521 | 66623 | 21/04/2025 | 30/04/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 522 | 66621 | 22/04/2025 | 09/05/2025 | LICENSE FEE RETAIL FRESH LICENCE | 3000 |
| 523 | 66464 | 22/04/2025 | 19/05/2025 | E-CHALLAN FOR GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 524 | 66602 | 22/04/2025 | 16/05/2025 | GRANT OF RETAIL & WHOLESALE DRUGS LICENSE | 6000 |
| 525 | 66378 | 22/04/2025 | 27/05/2025 | Fee for GRANT of wholesale drug license | 3000 |
| 526 | 66135 | 22/04/2025 | 03/06/2025 | Retail Drug License Fee | 3000 |
| 527 | 66636 | 22/04/2025 | 14/05/2025 | fee for the grant of retail drugs license | 3000 |
| 528 | 66637 | 22/04/2025 | 30/04/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 529 | 66638 | 22/04/2025 | 27/05/2025 | Fees for GRANT of retail and wholesale drug license | 6000 |
| 530 | 66341 | 22/04/2025 | 06/05/2025 | Fee for Grant of retail drug license | 3000 |
| 531 | 66644 | 22/04/2025 | 28/04/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 2998 |
| 532 | 66650 | 23/04/2025 | 28/05/2025 | HIMGRN NO. A25D126699 DATED ON 02.04.2025 | 3000 |
| 533 | 65854 | 23/04/2025 | 15/01/2025 | Constitution & PREMISES | 3000 |
| 534 | 66377 | 23/04/2025 | 09/05/2025 | HIMGRN Number A25C303942 on Dated 25.03.2025 [Fee submitted for the grant of Retail Sale Drugs License]. | 3000 |
| 535 | 66653 | 23/04/2025 | 30/04/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 536 | 66531 | 23/04/2025 | 30/04/2025 | Grant New Retail Drugs License Fee | 3000 |
| 537 | 66667 | 24/04/2025 | 15/05/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 538 | 66669 | 24/04/2025 | 30/04/2025 | A25D325257 on dated 21-04-2025 | 3000 |
| 539 | 66662 | 25/04/2025 | 02/05/2025 | LICENSE FEE RETAIL FRESH LICENCE | 3000 |
| 540 | 66630 | 25/04/2025 | 05/05/2025 | LICENSE FEE RETAIL FRESH LICENCE | 3000 |
| 541 | 66673 | 25/04/2025 | 20/05/2025 | Grant of Wholesale Drugs License | 3000 |
| 542 | 66677 | 25/04/2025 | 09/05/2025 | A25D237871 on dated 11-04-2025 | 3000 |
| 543 | 66679 | 25/04/2025 | 14/05/2025 | RETAIL DRUGS LICENSE | 3000 |
| 544 | 66453 | 25/04/2025 | 28/04/2025 | NEW REGISTRATION | 3000 |
| 545 | 66683 | 26/04/2025 | 19/05/2025 | Second application for grant of wholesale drugs license | 3000 |
| 546 | 66684 | 26/04/2025 | 01/05/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 547 | 66686 | 26/04/2025 | 06/05/2025 | RETENTION OF wholesale DRUG LICENSE | 3000 |
| 548 | 66685 | 26/04/2025 | 14/05/2025 | retail drugs store | 3000 |
| 549 | 66444 | 26/04/2025 | 30/06/2025 | Deposited through e-challan no. A25B2862741 dated 25.02.2025 | 3000 |
| 550 | 66692 | 26/04/2025 | 14/05/2025 | Fees for grant of retail of drug license | 3000 |
| 551 | 66697 | 28/04/2025 | 02/05/2025 | A25D142026 on dated 03-04-2025 | 3000 |
| 552 | 66695 | 28/04/2025 | 30/04/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 553 | 66702 | 28/04/2025 | 08/07/2025 | A25D390151 & CPAFBOCQM3 | 3000 |
| 554 | 66705 | 28/04/2025 | 02/05/2025 | ok New | 3000 |
| 555 | 66704 | 29/04/2025 | 14/05/2025 | Fees for grant of retail of drug license | 3000 |
| 556 | 66706 | 29/04/2025 | 27/05/2025 | Fees for grant of retail of drug license | 3000 |
| 557 | 66707 | 29/04/2025 | 28/05/2025 | Fee for Grant of Retail Drugs License | 3000 |
| 558 | 66304 | 29/04/2025 | 14/05/2025 | fee for fresh grant of retail drugs license | 3000 |
| 559 | 66712 | 30/04/2025 | 13/06/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 560 | 66714 | 30/04/2025 | 15/05/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 561 | 66715 | 30/04/2025 | 16/05/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 562 | 66719 | 30/04/2025 | 15/05/2025 | FEE FOR GRANT OF RETAIL & WHOLESALE DRUG LICENSE | 6000 |
| 563 | 66720 | 01/05/2025 | 30/05/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 564 | 66729 | 01/05/2025 | 30/05/2025 | Fee for the grant of Wholesale Drugs License. | 3000 |
| 565 | 66629 | 01/05/2025 | 19/05/2025 | E-CHALLAN FOR GRANT OF RETAIL & WHOLESALE DRUGS LICENSE | 6000 |
| 566 | 66738 | 02/05/2025 | 03/06/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 567 | 66645 | 02/05/2025 | 05/05/2025 | A25D327555 | 3000 |
| 568 | 66740 | 02/05/2025 | 09/05/2025 | RAMESH MEDICAL STORE | 3000 |
| 569 | 65679 | 03/05/2025 | 23/06/2025 | E-CHALLAN FOR GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 570 | 66694 | 03/05/2025 | 28/05/2025 | Fees for grant of retail of drug license | 3000 |
| 571 | 66524 | 06/05/2025 | 09/05/2025 | fee for addition of wholesale drug license to retail drug license | 3000 |
| 572 | 66747 | 06/05/2025 | 14/05/2025 | Fees For Fresh Wholesale Drug License | 3000 |
| 573 | 66750 | 06/05/2025 | 14/05/2025 | Fees For Fresh Retail Drug License | 3000 |
| 574 | 66752 | 06/05/2025 | 15/05/2025 | Grant of Wholesale License | 3000 |
| 575 | 66754 | 06/05/2025 | 26/05/2025 | Retail and Wholesale Drug License Fee | 6000 |
| 576 | 66751 | 06/05/2025 | 18/06/2025 | A25E148753 & CPAFCIQPU6 (Through online payment) | 3000 |
| 577 | 66757 | 06/05/2025 | 28/05/2025 | Retail and Wholesale Drug License Fee | 6000 |
| 578 | 66756 | 06/05/2025 | 14/05/2025 | PUSHAP MEDICAL STORE | 3000 |
| 579 | 66643 | 06/05/2025 | 08/07/2025 | Retail and Wholesale Drug License Fee | 6000 |
| 580 | 66762 | 06/05/2025 | 13/06/2025 | Retail and Wholesale Drug License Fee | 6000 |
| 581 | 66765 | 07/05/2025 | 24/06/2025 | LICENSE FEE FOR RETAIL | 3000 |
| 582 | 66743 | 07/05/2025 | 20/06/2025 | fees for grant of retail drug license | 3000 |
| 583 | 66777 | 08/05/2025 | 09/06/2025 | For Grant of wholesale drug license | 3000 |
| 584 | 66755 | 08/05/2025 | 19/05/2025 | A25D379495 on dated 26-04-2025 | 6000 |
| 585 | 66790 | 08/05/2025 | 16/05/2025 | FEE FOR WHOLESALE DRUG LICENSE | 3000 |
| 586 | 66792 | 08/05/2025 | 21/05/2025 | Fees For Fresh Retail and Wholesale Drug License | 6000 |
| 587 | 66799 | 09/05/2025 | 30/05/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 588 | 66796 | 09/05/2025 | 14/05/2025 | Fees for retention of Wholesale drugs license | 3000 |
| 589 | 66778 | 09/05/2025 | 03/06/2025 | fees for grant of retail drug license | 3000 |
| 590 | 66726 | 13/05/2025 | 28/05/2025 | HIMGRN NO. A25D357524 DATED ON 24.04.2025 | 3000 |
| 591 | 66816 | 13/05/2025 | 14/05/2025 | Fees For Fresh Retail Drug License | 3000 |
| 592 | 66818 | 13/05/2025 | 19/05/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 593 | 66772 | 13/05/2025 | 09/06/2025 | A25E115457 | 3000 |
| 594 | 66819 | 13/05/2025 | 02/06/2025 | FEE FOR GRAT OF WHOLESALE DRUG LICENSE | 3000 |
| 595 | 66820 | 13/05/2025 | 09/06/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 2999 |
| 596 | 66828 | 14/05/2025 | 12/06/2025 | A25E207215 & CPAFDACQR6 | 3000 |
| 597 | 66716 | 14/05/2025 | 23/05/2025 | HIMGRN Number A25D392530 on Dated 28.04.2025 [Fee submitted for the grant of Retail Sale Drugs License]. | 3000 |
| 598 | 66836 | 15/05/2025 | 09/06/2025 | HIMGRN NO. A25E198680 DATED ON 13.05.2025 | 3000 |
| 599 | 66808 | 15/05/2025 | 04/06/2025 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED VIDE HIMGRN NO B25E109668 DATED 15-05-2025 | 3000 |
| 600 | 66838 | 15/05/2025 | 28/05/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 601 | 66837 | 15/05/2025 | 26/06/2025 | Fee for Grant of retail drug license | 3000 |
| 602 | 66843 | 15/05/2025 | 04/06/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 603 | 66850 | 16/05/2025 | 28/05/2025 | FEE FOR GRANT OF RETAIL & WHOLESALE DRUG LICENSE | 6000 |
| 604 | 66851 | 16/05/2025 | 30/06/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 605 | 66853 | 16/05/2025 | 23/05/2025 | A25E215420 & CPAFDCOVQ4 | 6000 |
| 606 | 66857 | 16/05/2025 | 28/05/2025 | Deposited through e challan no. A25E149098 | 3000 |
| 607 | 66817 | 17/05/2025 | 23/05/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 608 | 66860 | 17/05/2025 | 04/06/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 609 | 65374 | 17/05/2025 | 26/03/2025 | GMP Certificate | 200 |
| 610 | 66419 | 19/05/2025 | 03/04/2025 | TEst payment details | 2000 |
| 611 | 66868 | 19/05/2025 | 10/06/2025 | Grant of Retail Drugs License | 3000 |
| 612 | 66870 | 19/05/2025 | 28/05/2025 | Fee for retail drugs license | 3000 |
| 613 | 66768 | 20/05/2025 | 30/05/2025 | Fee for Grant New Retail License | 3000 |
| 614 | 66768 | 20/05/2025 | 30/05/2025 | Fee for Grant New Retail License | 3000 |
| 615 | 66885 | 20/05/2025 | 09/06/2025 | NEW REGISTRATION | 3000 |
| 616 | 66888 | 20/05/2025 | 20/05/2025 | Grant of Retail D.L | 3000 |
| 617 | 66795 | 21/05/2025 | 23/05/2025 | New License | 3000 |
| 618 | 66831 | 21/05/2025 | 02/06/2025 | Retail and Wholesale Drug License Fee | 6000 |
| 619 | 66890 | 21/05/2025 | 28/05/2025 | Fee for retail drugs license | 3000 |
| 620 | 66766 | 22/05/2025 | 23/05/2025 | Fees for fresh retail drugs license | 3000 |
| 621 | 66903 | 22/05/2025 | 22/05/2025 | A25E250049 on dated 19-05-2025 | 2999 |
| 622 | 66884 | 22/05/2025 | 09/06/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 623 | 66916 | 23/05/2025 | 09/06/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 624 | 66907 | 23/05/2025 | 23/05/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 625 | 66918 | 23/05/2025 | 04/06/2025 | Fees for the grant of Retail drugs license | 3000 |
| 626 | 66929 | 23/05/2025 | 28/05/2025 | Fees For Fresh Retail Drug License | 3000 |
| 627 | 66930 | 24/05/2025 | 28/06/2025 | FOR WHOLESALE AND RETAIL | 6000 |
| 628 | 66830 | 24/05/2025 | 27/06/2025 | FEE FOR GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 629 | 66902 | 24/05/2025 | 26/06/2025 | RETAIL DRUGS LICENSE | 3000 |
| 630 | 66944 | 26/05/2025 | 27/05/2025 | FEE FOR THE WHOLESALE DRUGS LICENSE | 3000 |
| 631 | 66948 | 26/05/2025 | 10/06/2025 | Registration fee through UPI | 3000 |
| 632 | 66855 | 26/05/2025 | 03/06/2025 | FEE FOR THE RETAIL AND WHOLESALE DRUGS LICENSE | 6000 |
| 633 | 66945 | 26/05/2025 | 09/06/2025 | FEES of Rs 3000 SUBMITTED ONLINE VIDE HIMGRN NO A25D240538 DATED 11-04-2025 | 3000 |
| 634 | 66952 | 27/05/2025 | 01/07/2025 | New License | 3000 |
| 635 | 65504 | 27/05/2025 | 27/11/2024 | fee for the change in premises of Retail and Wholesale License. | 6000 |
| 636 | 66955 | 27/05/2025 | 28/05/2025 | Fees For Fresh Retail Drug License | 3000 |
| 637 | 66950 | 27/05/2025 | 03/06/2025 | Fee for Grant of retail drug license | 3000 |
| 638 | 66285 | 27/05/2025 | 28/06/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 639 | 65548 | 28/05/2025 | 27/11/2024 | Fee for change of premises | 3000 |
| 640 | 66962 | 28/05/2025 | 20/06/2025 | HIMGRN NO. A25C329978 DATED ON 27.03.2025 | 3000 |
| 641 | 66912 | 28/05/2025 | 12/06/2025 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED ONLINE VIDE HIMGRN NO A25E277185 DATED 22-05-2025 | 3000 |
| 642 | 66980 | 30/05/2025 | 23/06/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 643 | 66976 | 30/05/2025 | 24/06/2025 | A25E313925 | 3000 |
| 644 | 66967 | 31/05/2025 | 24/06/2025 | HIMGRN Number A25E318618 on Dated 28.05.2025 [Fee submitted for the grant of Retail Sale Drugs License]. | 3000 |
| 645 | 66196 | 02/06/2025 | 27/06/2025 | A25B112839 & CPAETQRBS3 | 500 |
| 646 | 66196 | 02/06/2025 | 27/06/2025 | A25B112839 & CPAETQRBS3 | 500 |
| 647 | 66196 | 02/06/2025 | 27/06/2025 | A25B112839 & CPAETQRBS3 | 500 |
| 648 | 66988 | 02/06/2025 | 01/07/2025 | Grant of Retail and Wholesale Drug License | 6000 |
| 649 | 66990 | 02/06/2025 | 09/06/2025 | NEW REGISTRATION | 3000 |
| 650 | 66917 | 02/06/2025 | 09/06/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 651 | 66995 | 03/06/2025 | 13/06/2025 | deposited through e-challan no. A25D241214 dated 11.04.2025 | 3000 |
| 652 | 66986 | 03/06/2025 | 28/06/2025 | Fee for the Grant of Retail Drugs License. | 3000 |
| 653 | 66997 | 03/06/2025 | 09/06/2025 | GRANT OFWHOLESALE DRUG LICENSE | 3000 |
| 654 | 66971 | 04/06/2025 | 20/06/2025 | HIMGRN NO. A24F 204342 DATED ON 17.06.2024 | 3000 |
| 655 | 66931 | 04/06/2025 | 25/06/2025 | fees for grant of retail drug license | 3000 |
| 656 | 67005 | 04/06/2025 | 09/06/2025 | New License | 3000 |
| 657 | 67013 | 05/06/2025 | 12/06/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 658 | 67015 | 05/06/2025 | 30/06/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 659 | 67014 | 06/06/2025 | 26/06/2025 | Fee for Grant of Retail Drugs License | 3000 |
| 660 | 67020 | 09/06/2025 | 24/06/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 661 | 67026 | 09/06/2025 | 28/06/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 662 | 67024 | 10/06/2025 | 18/06/2025 | LICENSE FEE FOR RETAIL | 3000 |
| 663 | 67028 | 10/06/2025 | 27/06/2025 | LICENSE FEE FOR RETAIL | 3000 |
| 664 | 66982 | 10/06/2025 | 12/06/2025 | NEW REGISTRATION | 3000 |
| 665 | 67033 | 10/06/2025 | 18/06/2025 | A25F177372 & CPAFFHLAU1 | 6000 |
| 666 | 67037 | 12/06/2025 | 24/06/2025 | A25F181523 & CPAFFILCE5 ( Through online payment) | 3000 |
| 667 | 66956 | 12/06/2025 | 24/06/2025 | Fees for fresh retail drugs license | 3000 |
| 668 | 67043 | 12/06/2025 | 27/06/2025 | Fees for GRANT of retail drug license | 3000 |
| 669 | 67040 | 12/06/2025 | 12/06/2025 | @@@@ | 1250 |
| 670 | 67044 | 12/06/2025 | 18/06/2025 | LICENSE FEE FOR RETENTION OF RETAIL AND WHOLE SALE | 6000 |
| 671 | 67047 | 12/06/2025 | 12/06/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 672 | 67046 | 12/06/2025 | 18/06/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 673 | 67040 | 12/06/2025 | 12/06/2025 | TEst | 125630 |
| 674 | 67040 | 12/06/2025 | 12/06/2025 | TEst | 20320 |
| 675 | 67040 | 12/06/2025 | 12/06/2025 | TEst | 12121212 |
| 676 | 67040 | 12/06/2025 | 12/06/2025 | dds | 2147483647 |
| 677 | 67040 | 12/06/2025 | 12/06/2025 | FDSF | 121 |
| 678 | 67057 | 12/06/2025 | 28/06/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 679 | 67056 | 12/06/2025 | 18/06/2025 | Fee for Fresh Wholesale Drugs License | 3000 |
| 680 | 67058 | 12/06/2025 | 23/06/2025 | Fees for fresh retail drugs license | 3000 |
| 681 | 67059 | 12/06/2025 | 28/06/2025 | FEES FOR GRANT OF WHOLESALE DRUGS LICENSE 3000 SUBMITTED ONLINE VIDE HIMGRN NO A25F201382 DATED 12-06-2025 | 3000 |
| 682 | 66748 | 13/06/2025 | 24/06/2025 | HIMGRN Number A25D229339 on Dated 17.04.2025 [Fee submitted for the grant of Retail Sale Drugs License]. | 3000 |
| 683 | 67075 | 16/06/2025 | 27/06/2025 | Deposited through e-challan no. A25E282688 dated 23.05.2025 | 3000 |
| 684 | 67072 | 16/06/2025 | 24/06/2025 | HIMGRN Number A25F224555 on Dated 16.06.2025 [Fee submitted for the grant of Retail Sale Drugs License]. | 3000 |
| 685 | 67080 | 17/06/2025 | 04/07/2025 | Fee for the Grant of Retail Drugs License. | 3000 |
| 686 | 67091 | 18/06/2025 | 18/06/2025 | Fees For Fresh Retail Drug License | 3000 |
| 687 | 67092 | 18/06/2025 | 18/06/2025 | Fees For Fresh Retail Drug License | 3000 |
| 688 | 67093 | 18/06/2025 | 01/07/2025 | LICENSE FEE OF RETAIL AND WHOLE SALE | 6000 |
| 689 | 67090 | 18/06/2025 | 24/06/2025 | Three thousand only | 3000 |
| 690 | 67098 | 18/06/2025 | 19/06/2025 | Fees For FRESH Wholesale Drug License | 3000 |
| 691 | 66994 | 19/06/2025 | 01/07/2025 | Grant of Wholesale Drug License | 3000 |
| 692 | 67103 | 19/06/2025 | 30/06/2025 | FEES FOR GRANT OF RETAIL DRUGS LICENSE 3000 SUBMITTED ONLINE. | 3000 |
| 693 | 67109 | 19/06/2025 | 01/07/2025 | Grant of retail drug license | 3000 |
| 694 | 67113 | 19/06/2025 | 26/06/2025 | Fee for Grant of wholesale drug license | 3000 |
| 695 | 66964 | 19/06/2025 | 30/06/2025 | FEE FOR GRANT OF WHOLESALE DRUGS LICENSE | 3000 |
| 696 | 67111 | 19/06/2025 | 07/07/2025 | Grant of Retail Drugs License | 3000 |
| 697 | 66933 | 20/06/2025 | 30/06/2025 | FEE FOR GRANT OF RETAIL DRUGS LICENSE | 3000 |
| 698 | 67130 | 21/06/2025 | 27/06/2025 | Fees for fresh of Retail & Wholesale drugs license | 6000 |
| 699 | 67135 | 21/06/2025 | 30/06/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 700 | 67136 | 21/06/2025 | 05/07/2025 | A25F268864 & CPAFGNIIP3 | 6000 |
| 701 | 67142 | 22/06/2025 | 25/06/2025 | fee for fresh grant of retail drugs license | 3000 |
| 702 | 66975 | 23/06/2025 | 26/06/2025 | Fee for grant of whole sale drug licence | 3000 |
| 703 | 67148 | 24/06/2025 | 24/06/2025 | Retail | 3000 |
| 704 | 67155 | 25/06/2025 | 04/07/2025 | Deposited Through e-challan no. A25F116065 dated 03.06.2025 | 3000 |
| 705 | 67089 | 26/06/2025 | 27/06/2025 | FEE FOR GRANT OF RETAIL DRUG LICENCE | 3000 |
| 706 | 67184 | 27/06/2025 | 07/07/2025 | FEE FOR GRANT OF WHOLESALE DRUG LICENSE | 3000 |
| 707 | 67127 | 27/06/2025 | 30/06/2025 | Paid through e challan | 3000 |
| 708 | 66013 | 27/06/2025 | 05/02/2025 | Fee for Change of premises of Retail & Wholesale drug license | 6000 |
| 709 | 66287 | 30/06/2025 | 28/03/2025 | FEE FOR CHANGE IN PREMIES OF WHOLESALE DRUG LICENSE | 3000 |
| 710 | 67152 | 03/07/2025 | 07/07/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
| 711 | 67259 | 04/07/2025 | 08/07/2025 | New License | 3000 |
| 712 | 67225 | 04/07/2025 | 08/07/2025 | A25E305748 dated 26-05-2025 | 3000 |
| 713 | 67209 | 04/07/2025 | 08/07/2025 | A25F306270 dated 24-06-2025 | 3000 |
| 714 | 66753 | 04/07/2025 | 08/07/2025 | B25D100312 on dated 01-04-2025 | 3000 |
| 715 | 67266 | 05/07/2025 | 07/07/2025 | FEE FOR GRANT OF RETAIL DRUG LICENSE | 3000 |
25 day(s) 10 hr(s)
18 day(s) 21 hr(s)
0 day(s) 0 hr(s)
168 day(s) 1 hr(s)
259
| Sr.No. | Circle | Applications Received | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | ADC Office, Solan (BBN) |
176 ADC Office, Solan (BBN)
Applications Received: 176
|
||||||
| 2 | ADC Office, Solan |
121 ADC Office, Solan
Applications Received: 121
|
||||||
| 3 | ADC Office, Sirmaur |
108 ADC Office, Sirmaur
Applications Received: 108
|
||||||
| 4 | ADC Office, Chamba |
8 ADC Office, Chamba
Applications Received: 8
|
||||||
| 5 | ADC Office, Kangra |
18 ADC Office, Kangra
Applications Received: 18
|
||||||
| 6 | ADC Office, Una |
10 ADC Office, Una
Applications Received: 10
|
||||||
| 7 | ADC Office, Bilaspur |
2 ADC Office, Bilaspur
Applications Received: 2
|
||||||
| 8 | ADC Office, Shimla |
1 ADC Office, Shimla
Applications Received: 1
|
||||||
| 9 | ADC Office, Hamirpur |
2 ADC Office, Hamirpur
Applications Received: 2
|
||||||
| 10 | ADC Office, Kullu |
1 ADC Office, Kullu
Applications Received: 1
|
||||||
| 11 | ADC Office, Mandi |
1 ADC Office, Mandi
Applications Received: 1
|
15
18
154
₹ 427977.76
| Sr.No. | Application No | Application Date | Approval Date | Fee Detail | Total Fee Charged |
|---|---|---|---|---|---|
| 1 | 5 | 06/11/2024 | 08/10/2024 | Retention of License | 15000 |
| 2 | 5 | 06/11/2024 | 08/10/2024 | Fee FOR Retention OF Additional Products | 202000 |
| 3 | 8 | 06/11/2024 | 08/10/2024 | A24J156562 | 15000 |
| 4 | 6 | 06/11/2024 | 08/10/2024 | FEES FOR RETENTION OF LOAN LICENCE AND PRODUCT | 8500 |
| 5 | 8 | 06/11/2024 | 08/10/2024 | A24J156562 | 15000 |
| 6 | 10 | 06/11/2024 | 10/10/2024 | E CHALLAN | 15000 |
| 7 | 11 | 06/11/2024 | 22/11/2024 | Dwarka Global Lifescience to State Drugs Controller Baddi | 15000 |
| 8 | 14 | 06/11/2024 | 17/10/2024 | DRUG MANUFACTURER -Fees for retention of drugs mfg Loan License | 16200 |
| 9 | 12 | 06/11/2024 | 17/10/2024 | Retention of Drug Manufacturing Loan License and Retention Products | 17500 |
| 10 | 16 | 06/11/2024 | 17/10/2024 | Fees for retention of drugs mfg Loan License | 15000 |
| 11 | 17 | 06/11/2024 | 17/10/2024 | DRUG MANUFACTURER -Fees for retention of drugs mfg Loan License | 15900 |
| 12 | 18 | 06/11/2024 | 18/10/2024 | Online E-Challan Fee for Retention of Drugs Manufacturing License MNB/09/746 & MB/09/747 (Maneesh Pharmaceuticals Ltd. | 15000 |
| 13 | 21 | 06/11/2024 | 21/02/2025 | Treasury No: SOL05 / DDO-019 / State Drug Controller | 15000 |
| 14 | 26 | 06/11/2024 | 24/10/2024 | Rs. 15000 Fee for Retention of Manufacturing License and Rs. 240000 for Retention of production permissions | 255000 |
| 15 | 29 | 06/11/2024 | 25/10/2024 | Fee of Retention of Loan License (BM Pharmaceuticals C/o Scott-Edil Pharmacia Ltd., Unit-II, Plot No. 21-22, EPIP, Phase-I, Jharmajri, Badii, Distt. Solan, H.P.) | 15000 |
| 16 | 29 | 06/11/2024 | 25/10/2024 | Renewal fees of BM Pharmaceuticals C/o Scott-Edil Pharmacia Ltd. Unit-2, Plot No. 21-22, EPIP, Phase-I, Jharmajri, Baddi, Distt. Solan, H.P. | 15000 |
| 17 | 15 | 06/11/2024 | 03/03/2025 | Fees for Application of Loan licence of Abbott Healthcare Pvt. Ltd. c/o Acme Generics Pvt. Ltd (Form 25 A and 28 A) | 15000 |
| 18 | 28 | 06/11/2024 | 09/12/2024 | CPAEGOVRR3 | 15000 |
| 19 | 32 | 06/11/2024 | 04/11/2024 | DRUG MANUFACTURER -Fees for retention of drugs mfg Loan License | 7500 |
| 20 | 34 | 06/11/2024 | 07/11/2024 | Deposted thorugh two challan Bank Refrence no. 214164328898 & 21416401614 | 15000 |
| 21 | 22 | 06/11/2024 | 14/01/2025 | FEE FOR LOAN LICENSE | 15000 |
| 22 | 47 | 16/11/2024 | 20/12/2024 | FIFTEEN THOUSAND RUPEES ONLY | 15000 |
| 23 | 42 | 16/11/2024 | 18/12/2024 | HIMKOSH -ONLINE | 15000 |
| 24 | 25 | 20/11/2024 | 25/11/2024 | PAID ON 08TH OCTOBER 2024, SOFT COPY ATTACHED | 7500 |
| 25 | 54 | 22/11/2024 | 22/11/2024 | RENEWAL FEES | 15000 |
| 26 | 39 | 22/11/2024 | 05/12/2024 | For Grant of Loan License | 7500 |
| 27 | 24 | 23/11/2024 | 23/11/2024 | Renewal of Manufacturing Loan License | 15000 |
| 28 | 63 | 28/11/2024 | 02/12/2024 | CHIEF MEDICAL OFFICER NAHAN | 15000 |
| 29 | 62 | 28/11/2024 | 15/01/2025 | Drug Manufacturer-Loan Licence of FLORAKIND PHARMACEUTICALS at Star Filtration Systems, Kala-Amb | 30000 |
| 30 | 63 | 02/12/2024 | 02/12/2024 | CHIEF MEDICAL OFFICER NAHAN | 15000 |
| 31 | 69 | 03/12/2024 | 03/12/2024 | Fees for retention of drugs mfg Loan License | 16500 |
| 32 | 67 | 03/12/2024 | 05/12/2024 | Transation Success of e challan A24K312611 DDO Chief Medical Officer Nahan | 7500 |
| 33 | 64 | 04/12/2024 | 17/01/2025 | Rs 7500/- paid to State Drug Controller Baddi and Rs 7500/- deposited to Society for Strengthening of Drug Regulatory System | 15000 |
| 34 | 64 | 04/12/2024 | 17/01/2025 | Rs 7500 paid to State Drug Controller Baddi and Rs 7500 deposited to Society for Strengthening of Drug Regulatory System | 15000 |
| 35 | 76 | 05/12/2024 | 05/12/2024 | Deposited Through e challan no. A24L104020 and Facilatation fees Society for Strengthening of Drugs Regulating Systems of Rs. 15000 vide NEFT 038459865111 | 15000 |
| 36 | 29 | 05/12/2024 | 25/10/2024 | Retention fees of Drug Manufacturing Loan License- BM Pharmaceuticals C/o Scott-Edil Pharmacia Ltd. Unit-2 at 21-22, EPIP, Phase-1, Jharmajri, Baddi, Distt. Solan, H.P. | 15000 |
| 37 | 72 | 05/12/2024 | 11/04/2025 | Mankind Pharma Limited C/o Higgs Healthcare LL Application Fees & Facilitation Fees | 7500 |
| 38 | 80 | 06/12/2024 | 06/12/2024 | Retention of mfg license copy | 7500 |
| 39 | 77 | 07/12/2024 | 07/12/2024 | ONLINE | 15000 |
| 40 | 73 | 07/12/2024 | 10/01/2025 | Fees for Form24A application M/s Alkem Laboratories Ltd C/o Acme Generics Pvt Ltd | 7500 |
| 41 | 82 | 07/12/2024 | 14/02/2025 | fees for Drugs manufacturing license | 15000 |
| 42 | 82 | 08/12/2024 | 14/02/2025 | fees for drugs manufacturing license | 15000 |
| 43 | 82 | 08/12/2024 | 14/02/2025 | fees for drugs manufacturing license | 15000 |
| 44 | 87 | 08/12/2024 | 22/03/2025 | Fees for Schedule X License | 20000 |
| 45 | 59 | 10/12/2024 | 03/02/2025 | Fee for Grant of Loan License | 15000 |
| 46 | 85 | 10/12/2024 | 10/12/2024 | 21608428173 | 7650 |
| 47 | 84 | 13/12/2024 | 13/12/2024 | 7500 payment for Retention of Drug Manufacturing License | 15000 |
| 48 | 56 | 13/12/2024 | 13/12/2024 | State Drugs Controller Baddi | 30000 |
| 49 | 83 | 13/12/2024 | 21/12/2024 | bank reference no. 21811903667 | 15000 |
| 50 | 77 | 13/12/2024 | 07/12/2024 | A24L187972, BANK REF NO. 21841914752 | 146000 |
| 51 | 86 | 13/12/2024 | 13/12/2024 | Drug Manufacturer-Product Approval Fees | 90000 |
| 52 | 77 | 14/12/2024 | 07/12/2024 | A24L193315, bANK REF NO. 21851348585 | 1500 |
| 53 | 93 | 14/12/2024 | 25/03/2025 | FOR GRANT OF DRUG MANUFACTURING LICENCE | 15000 |
| 54 | 42 | 17/12/2024 | 18/12/2024 | online | 18000 |
| 55 | 98 | 17/12/2024 | 26/03/2025 | DONE | 15000 |
| 56 | 42 | 18/12/2024 | 18/12/2024 | HIMKOSH -ONLINE., PNB -ONLINE | 30000 |
| 57 | 95 | 18/12/2024 | 21/12/2024 | FEES FOR GRANT OF DRUGS MFG LOAN LICENCES | 15000 |
| 58 | 43 | 19/12/2024 | 04/03/2025 | FEE FOR GRANT LOAN LICENCE AND SFSODR | 15000 |
| 59 | 102 | 19/12/2024 | 19/12/2024 | deposited SSDSR (Facitation) fees Rs 15000 vide number FT411212800708 dated 22.11.2024 for retention of Manufacturing licenses | 15000 |
| 60 | 96 | 19/12/2024 | 28/01/2025 | Fee for Loan License of Balaji Innovvative Solution. | 7500 |
| 61 | 101 | 20/12/2024 | 16/01/2025 | A24L230870 | 15000 |
| 62 | 106 | 20/12/2024 | 20/12/2024 | Subitted Retention fees Rs. 15000 vide e-challan no. A24K307125, Facilitation fees SSDRS vide NEFT no. M459987 and products retention fees Rs 160000 vide E-challan no. A24K307158 | 15000 |
| 63 | 109 | 21/12/2024 | 16/01/2025 | A24l228306 , 21890914558 | 15000 |
| 64 | 116 | 24/12/2024 | 23/01/2025 | Fees for Grant of Drugs Mfg Loan Licence | 30000 |
| 65 | 117 | 24/12/2024 | 06/01/2025 | CPAEOTXXH6 | 15000 |
| 66 | 113 | 24/12/2024 | 24/12/2024 | Online payment of 15000 and 300 by GPAY and 15300 for Society for strengthening of Drug Regulatory system by NEFT transfer | 30600 |
| 67 | 83 | 27/12/2024 | 21/12/2024 | FEE FOR RETENTION OF LICENSE | 95700 |
| 68 | 55 | 28/12/2024 | 28/12/2024 | A24L155230 | 15000 |
| 69 | 114 | 30/12/2024 | 04/02/2025 | FEES FOR GRANT OF DRUGS MFG LOAN LICENCES | 15000 |
| 70 | 113 | 31/12/2024 | 24/12/2024 | Online payment of 15000 and 300 by GPAY and 15300 for Society for strengthening of Drug Regulatory system by NEFT transfer | 30600 |
| 71 | 84 | 31/12/2024 | 13/12/2024 | 7500 payment for Retention of Drug Manufacturing License In Society Account | 7500 |
| 72 | 115 | 31/12/2024 | 11/02/2025 | Fees for Form 24-A and Form 27-A | 15000 |
| 73 | 125 | 31/12/2024 | 06/03/2025 | 15000 in DRUGREGULATORY Reference ID: 641223683 Beneficiary Account number: 4131000100478603 | 15000 |
| 74 | 131 | 02/01/2025 | 14/01/2025 | Grant of Drugs Manufacturing Loan Licence | 30000 |
| 75 | 140 | 03/01/2025 | 07/03/2025 | Fee for Loan License | 15000 |
| 76 | 77 | 04/01/2025 | 07/12/2024 | Society for strengthening of drugs regulatory system | 15000 |
| 77 | 139 | 04/01/2025 | 29/01/2025 | FEE FOR THE GRANT OF DRUGS MANUFACTURING LOAN LICENSE | 15000 |
| 78 | 55 | 06/01/2025 | 28/12/2024 | Zuvius lifesciences Pvt.Ltd C/o Beta Drugs lTD | 15000 |
| 79 | 123 | 06/01/2025 | 06/01/2025 | CIN NUMVER 21947314825 | 15000 |
| 80 | 123 | 06/01/2025 | 06/01/2025 | CPAEPOFRV5 | 10000 |
| 81 | 138 | 06/01/2025 | 15/02/2025 | payment against e challan | 15000 |
| 82 | 71 | 06/01/2025 | 20/12/2024 | Attached both details Govt. Treasury and Facilitation charges | 30000 |
| 83 | 62 | 08/01/2025 | 15/01/2025 | DRUG MANUFACTURER -GRANT OF DRUGS MANUFACTURING LOAN LICENCE | 15000 |
| 84 | 62 | 08/01/2025 | 15/01/2025 | Drug Manufacturer-Loan Licence of FLORAKIND PHARMACEUTICALS at Star Filtration Systems, Kala-Amb SSDRS | 15000 |
| 85 | 144 | 09/01/2025 | 27/01/2025 | Sucessful | 15000 |
| 86 | 135 | 09/01/2025 | 03/03/2025 | Both the payments (Govt. Treasury & Facilitation) attached | 7500 |
| 87 | 103 | 09/01/2025 | 28/01/2025 | E CHALLAN Rs 7500,Society of Strengthening of Drug Regulatory System Rs 7500 | 15000 |
| 88 | 143 | 10/01/2025 | 03/03/2025 | Fee Chalan For Form 27A | 7500 |
| 89 | 151 | 11/01/2025 | 18/02/2025 | Fees for Form 24-A | 7500 |
| 90 | 149 | 13/01/2025 | 03/02/2025 | SUCCESSFULLY | 15000 |
| 91 | 146 | 14/01/2025 | 05/03/2025 | PAYMENT FOR GRANT OF DRUG MANUFACTURING LICENSE | 15000 |
| 92 | 156 | 15/01/2025 | 18/02/2025 | Fees for FORM 24A and FORM 27A | 15000 |
| 93 | 149 | 15/01/2025 | 03/02/2025 | Sucessful | 15000 |
| 94 | 132 | 15/01/2025 | 20/02/2025 | RETENTION FOR LOAN LICENCE FEES | 15000 |
| 95 | 60 | 17/01/2025 | 20/02/2025 | Net Banking | 7500 |
| 96 | 158 | 17/01/2025 | 04/02/2025 | FEES FOR GRANT LOAN LICENCE 15000, NEFT FROM BANK SBI 15000 | 30000 |
| 97 | 155 | 18/01/2025 | 01/04/2025 | Sucessful | 15000 |
| 98 | 77 | 20/01/2025 | 07/12/2024 | Retention product's approval fees | 116100 |
| 99 | 163 | 20/01/2025 | 20/01/2025 | Both Govt Treasury (HIMKOSH) & Facilation charges payment done and receipt attached | 30000 |
| 100 | 165 | 21/01/2025 | 21/01/2025 | A34AZ35234 | 15000 |
| 101 | 172 | 23/01/2025 | 10/02/2025 | Loan License File Samarth Life sciences Private limited Care of KKasin Life sciences | 15000 |
| 102 | 164 | 24/01/2025 | 17/02/2025 | Drug Manufacture Fee for Drug Manufacturing License | 15000 |
| 103 | 176 | 25/01/2025 | 04/02/2025 | fees for Drugs manufacturing Loan license | 15000 |
| 104 | 170 | 25/01/2025 | 24/01/2025 | Drug Retention of loan license , Society strengthening of drug | 23000 |
| 105 | 174 | 25/01/2025 | 25/01/2025 | Fee for retention & Additional products | 510000 |
| 106 | 166 | 26/01/2025 | 04/02/2025 | fees for Drugs manufacturing Loan license | 15000 |
| 107 | 180 | 26/01/2025 | 22/05/2025 | CHALLAN FOR NEW DRUG LICENSE | 15000 |
| 108 | 113 | 27/01/2025 | 24/12/2024 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 Trasaction Description ClCN520250120003 73651/and transaction ID S85036516 | 30000 |
| 109 | 76 | 28/01/2025 | 05/12/2024 | DRUG MANUFACTURING PRODUCT RENEWAL FEES | 30000 |
| 110 | 157 | 28/01/2025 | 01/04/2025 | 0210-01-107-01(DRUG MANUFACTURER)STATE DRUGS CONTROLLER BADDI and Facility Charge UTR NO. N365243479221457Society for Strengthening of Drugs Regulatory System | 30000 |
| 111 | 164 | 28/01/2025 | 17/02/2025 | Fee for grant of loan license in drug Strenthening account | 15000 |
| 112 | 81 | 28/01/2025 | 28/01/2025 | FEE FOR RETENTION OF PRODUCT PERMISSION | 41400 |
| 113 | 81 | 28/01/2025 | 28/01/2025 | FEE FOR RETENTION OF PRODUCT PERMISSION | 41400 |
| 114 | 81 | 28/01/2025 | 28/01/2025 | FEE FOR RETENTION OF MANUFACTURING LICENSES (FORM-25 & FORM-28) | 15000 |
| 115 | 81 | 28/01/2025 | 28/01/2025 | FEE FOR RETENTION OF MANUFACTURING LICENSES (FORM-25 & FORM-28) | 15000 |
| 116 | 183 | 28/01/2025 | 10/02/2025 | Loan License File Samarth Life Science C/o Tridev Bio Sciences | 35000 |
| 117 | 147 | 29/01/2025 | 29/01/2025 | A25A317121 | 7500 |
| 118 | 147 | 29/01/2025 | 29/01/2025 | A25A317121 | 7500 |
| 119 | 185 | 29/01/2025 | 27/02/2025 | Sucessful | 15000 |
| 120 | 102 | 29/01/2025 | 19/12/2024 | Fee for retention of Drugs manufacturing Licenses Form 25 and 28 | 15000 |
| 121 | 113 | 30/01/2025 | 24/12/2024 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 transaction ID S85036516 | 30000 |
| 122 | 188 | 30/01/2025 | 14/02/2025 | UTR-873638412153 | 30000 |
| 123 | 107 | 31/01/2025 | 28/01/2025 | Renewal of Manufacturing Loan License and products | 8100 |
| 124 | 132 | 31/01/2025 | 20/02/2025 | FEES FOR RETAINTION FOR DRUG MANUFACTURING LOAN LICENCES | 7500 |
| 125 | 173 | 31/01/2025 | 14/02/2025 | Grant of Manufacturing Loan License (Payment details of HIMKOSH and Society for SDRS) | 7500 |
| 126 | 118 | 01/02/2025 | 16/04/2025 | Payment for Form 24A & Form 27A & Society Challan | 15000 |
| 127 | 121 | 01/02/2025 | 01/02/2025 | Depsoited through E-challan no. A24L261917 | 7500 |
| 128 | 182 | 03/02/2025 | 07/03/2025 | STATE DRUGS CONTROLLER BADDI | 15000 |
| 129 | 113 | 04/02/2025 | 24/12/2024 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 transaction ID S85036516 | 30000 |
| 130 | 136 | 04/02/2025 | 27/03/2025 | For Loan License | 15000 |
| 131 | 195 | 04/02/2025 | 13/03/2025 | FEE FOR GRANT OF LOAN ON FORM-28A (e-Challan Rs. 7500 + SSDRS Rs.7500) | 7500 |
| 132 | 113 | 05/02/2025 | 24/12/2024 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 transaction ID S85036516 | 30000 |
| 133 | 113 | 05/02/2025 | 24/12/2024 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 transaction ID S85036516 | 30000 |
| 134 | 198 | 06/02/2025 | 01/03/2025 | Grant of Manufacturing Loan License (Payment details of HIMKOSH and Society for SDRS) | 15000 |
| 135 | 191 | 06/02/2025 | 15/02/2025 | ONLINE | 15000 |
| 136 | 118 | 06/02/2025 | 16/04/2025 | Payment for Form 25A & Form 28A | 15000 |
| 137 | 83 | 07/02/2025 | 21/12/2024 | PAYMENT FOR DRUG RETENTION | 15000 |
| 138 | 196 | 07/02/2025 | 01/03/2025 | FDC Limited C/o Prosperity Drugs Pvt. Ltd | 7500 |
| 139 | 178 | 07/02/2025 | 25/03/2025 | Loan License Fee | 15000 |
| 140 | 203 | 07/02/2025 | 07/02/2025 | Net Banking | 15000 |
| 141 | 121 | 07/02/2025 | 01/02/2025 | Deposited Through e challan no. A24L262027 | 7500 |
| 142 | 201 | 08/02/2025 | 08/02/2025 | A25B143015 | 14998 |
| 143 | 207 | 08/02/2025 | 08/02/2025 | Retention of Drugs Manufacturing License | 15000 |
| 144 | 204 | 08/02/2025 | 19/03/2025 | LOAN LICENCES FEES | 15000 |
| 145 | 160 | 08/02/2025 | 27/03/2025 | FEES FOR GRANT OF DRUGS MANUFACTURING LICENCE | 7500 |
| 146 | 186 | 08/02/2025 | 30/01/2025 | Fee for Retention of Loan License & Late Fees | 15900 |
| 147 | 186 | 08/02/2025 | 30/01/2025 | Fee for Retention of Loan License & Late Fees | 15900 |
| 148 | 186 | 10/02/2025 | 30/01/2025 | Renewal fees & Late fees | 15900 |
| 149 | 208 | 10/02/2025 | 19/03/2025 | Payments for Grant of Loan License | 15000 |
| 150 | 209 | 10/02/2025 | 10/02/2025 | For Retention of Manufacturing License | 15000 |
| 151 | 187 | 10/02/2025 | 04/04/2025 | Fees paid for loan license Lupin Ltd c/o Pontika Aerotech Limited | 7500 |
| 152 | 210 | 11/02/2025 | 04/03/2025 | Grant of Manufacturing Loan License (Payment details of HIMKOSH and Society for SDRS) | 15000 |
| 153 | 113 | 11/02/2025 | 24/12/2024 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 transaction ID S85036516 | 30000 |
| 154 | 211 | 11/02/2025 | 11/02/2025 | ONLINE PAYMENT | 15000 |
| 155 | 214 | 13/02/2025 | 01/04/2025 | Deposited through E-challan no. A25B177467 | 7500 |
| 156 | 219 | 13/02/2025 | 04/03/2025 | Grant of Manufacturing Loan License (Payment details of HIMKOSH and Society for SDRS) | 15000 |
| 157 | 220 | 14/02/2025 | 04/03/2025 | FEE FOR GRANT OF DRUG LICENCE | 7500 |
| 158 | 196 | 14/02/2025 | 01/03/2025 | Society for strengthening of Drugs Regulatory System | 7500 |
| 159 | 113 | 15/02/2025 | 24/12/2024 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 transaction ID S85036516 | 30000 |
| 160 | 191 | 15/02/2025 | 15/02/2025 | SOCIETY PAYMENT RECIEPT | 15000 |
| 161 | 226 | 17/02/2025 | 22/04/2025 | Fee For Grant of LL | 15000 |
| 162 | 113 | 18/02/2025 | 24/12/2024 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 transaction ID S85036516 | 30000 |
| 163 | 223 | 18/02/2025 | 18/02/2025 | Retention of Loan Licence of M/s Impact Healthcare Pvt. Ltd C/o M/s Associated Biotech | 15000 |
| 164 | 222 | 18/02/2025 | 24/04/2025 | FEE FOR THE GRANT OF DRUGS MANUFACTURING LICENSE | 30000 |
| 165 | 212 | 19/02/2025 | 03/04/2025 | chief medical Officer,Nahan | 15000 |
| 166 | 160 | 19/02/2025 | 27/03/2025 | FEES FOR GRANT OF DRUGS MANUFACTURING LICENSE | 7500 |
| 167 | 221 | 19/02/2025 | 17/02/2025 | E-Challan Payment 7500.00 rupees vide Chalan No : A25B133449 dated 05/02/2025 , E-Challan Payment 28000.0 rupees vide Challan No: A25B179588 dated 12/02/2025 , Rupees 7500.0 Payment Paid to Drugs Regulator vide UTR No.: S36394151 dated 05/02/2025. , R | 71000 |
| 168 | 229 | 19/02/2025 | 19/02/2025 | Deposited Through E Challan No. A25B243526 and Facilitation fees Rs 15000 vide reference id no. M969893 dated 19.02.2025 | 15000 |
| 169 | 228 | 19/02/2025 | 24/03/2025 | PAID ONLINE | 15000 |
| 170 | 60 | 20/02/2025 | 20/02/2025 | Challan Fees and Society Fees for retention of Loan License ( 7500+ 7500) | 7500 |
| 171 | 132 | 20/02/2025 | 20/02/2025 | HIMKOSH-15000, SOCIETY-7500 | 22500 |
| 172 | 202 | 22/02/2025 | 22/02/2025 | HIMKOSH CHALLAN NO A25A335593 ON DATED 29.01.2025 AND SFSODRS UTR NO. SBIN125027983854 ON DATED 27.01.2025 | 15000 |
| 173 | 113 | 22/02/2025 | 24/12/2024 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 transaction ID S85036516 | 30000 |
| 174 | 234 | 24/02/2025 | 24/02/2025 | A25B254624 , A25B254654 & SBIN42505495227 | 280000 |
| 175 | 232 | 24/02/2025 | 24/02/2025 | Deposited through E-challan no. A25B262030 dated 21.02.2025 and Facilitation fees vide NEFT Reference no. N52025022100507018 dated 21.02.2025 | 15000 |
| 176 | 232 | 24/02/2025 | 24/02/2025 | Deposited through E-challan no. A25B262030 dated 21.02.2025 and Facilitation fees vide NEFT Reference no. N52025022100507018 dated 21.02.2025 | 15000 |
| 177 | 167 | 24/02/2025 | 06/03/2025 | PAYMENT ONLINE FROM THE NAME OF AASHIMA SHARMA ( PAYMENT AMOUNT 15000+15000) | 30000 |
| 178 | 213 | 25/02/2025 | 24/04/2025 | NEFT FROM HDFC | 30000 |
| 179 | 233 | 25/02/2025 | 25/02/2025 | DRUG MANUFACTURER -Retention of Drug Manufacturing License | 15000 |
| 180 | 113 | 27/02/2025 | 24/12/2024 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 transaction ID S85036516 | 30000 |
| 181 | 122 | 27/02/2025 | 28/03/2025 | CREDIT CARD,PAY TM | 30000 |
| 182 | 119 | 27/02/2025 | 27/02/2025 | Facilitation charges also done with UTR NO: SBIN325058167578 | 15000 |
| 183 | 237 | 28/02/2025 | 28/02/2025 | A25B292756 | 15000 |
| 184 | 181 | 28/02/2025 | 28/02/2025 | Fee for Retention of Loan License of Glenmark Pharma c/o Metrocraft Baddi HP | 15000 |
| 185 | 240 | 28/02/2025 | 28/02/2025 | FEE FOR THE RETENTION OF LOAN LICENSE WITH PRODUCTS | 14100 |
| 186 | 148 | 28/02/2025 | 28/02/2025 | Rentention Fee for Drugs Licesne | 30000 |
| 187 | 148 | 28/02/2025 | 28/02/2025 | Rentention Fee for Drugs Licesne | 30000 |
| 188 | 242 | 28/02/2025 | 28/02/2025 | ONLINE | 15000 |
| 189 | 243 | 01/03/2025 | 01/03/2025 | Fees for Retention of Drug manufacturing Licence , Fees for retention products | 96000 |
| 190 | 133 | 03/03/2025 | 07/04/2025 | OK | 30000 |
| 191 | 246 | 03/03/2025 | 03/03/2025 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 transaction ID S85036516 | 30000 |
| 192 | 113 | 03/03/2025 | 24/12/2024 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 transaction ID S85036516 | 30000 |
| 193 | 148 | 04/03/2025 | 28/02/2025 | Rentention Fee for Drugs Licesne | 30000 |
| 194 | 132 | 04/03/2025 | 20/02/2025 | HIMKOSH-7500 & Society for Strengthenin -7500 | 15000 |
| 195 | 244 | 04/03/2025 | 04/03/2025 | FEE FOR RETENTION DRUGS LICENCE | 15000 |
| 196 | 251 | 05/03/2025 | 05/03/2025 | FEE FOR LOAN LICENSE Retention | 7500 |
| 197 | 253 | 05/03/2025 | 05/03/2025 | HIMKOSH-7500/- & Society for STRENGTHENING-7500/- | 15000 |
| 198 | 113 | 06/03/2025 | 24/12/2024 | Online Payment of 15000 by Net banking through Himkosh and online payment of Rs.15000 to account No. 000905001483 transaction ID S85036516 | 30000 |
| 199 | 255 | 06/03/2025 | 06/03/2025 | FEE FOR RETENTION OF MANUFACTURING LICENSE | 30000 |
| 200 | 245 | 07/03/2025 | 05/03/2025 | A25B302066 & INDBH28023204618 | 30000 |
| 201 | 211 | 07/03/2025 | 11/02/2025 | Society for strengthening of drugs | 15000 |
| 202 | 248 | 08/03/2025 | 08/03/2025 | Challan submitted to both 7500 in himkosh & 7500 on facilitation charge ref no. 503043024469TRF on Dated 04/03/2025 HDFC bank | 7500 |
| 203 | 249 | 08/03/2025 | 11/04/2025 | Net Banking | 7500 |
| 204 | 241 | 08/03/2025 | 11/04/2025 | Grant for Loan License | 15000 |
| 205 | 256 | 08/03/2025 | 31/03/2025 | DRUG MANUFACTURER -fee for Renewal of Drugs Mfg Licence | 15000 |
| 206 | 261 | 10/03/2025 | 12/03/2025 | Grant of Manufacturing Loan License (Payment details of HIMKOSH and Society for SDRS) | 15000 |
| 207 | 236 | 10/03/2025 | 10/03/2025 | license renewal fee | 15600 |
| 208 | 254 | 11/03/2025 | 07/03/2025 | Transation Success of e challan A25C175950, DDO Chief Medical Officer Nahan of amount 15000 | 15000 |
| 209 | 260 | 11/03/2025 | 11/03/2025 | M0210000348 | 15000 |
| 210 | 31 | 12/03/2025 | 10/03/2025 | E-CHALLAN, NEFT | 30000 |
| 211 | 262 | 12/03/2025 | 12/03/2025 | Retention of Manufacturing License (Payment details of HIMKOSH and Society for SDRS) | 15000 |
| 212 | 148 | 12/03/2025 | 28/02/2025 | Rentention Fee for Drugs Licesne | 30000 |
| 213 | 238 | 12/03/2025 | 12/03/2025 | Fee for Retention of Drugs Manufacturing License of Small Volume Parenterals (SVP) MB/09/826 | 7500 |
| 214 | 129 | 12/03/2025 | 12/03/2025 | DRUG MANUFACTURER - RETENTION OF DRUG MANUFACTURING LICENCE | 15000 |
| 215 | 31 | 13/03/2025 | 10/03/2025 | E-CHALLAN, NEFT | 30000 |
| 216 | 260 | 13/03/2025 | 11/03/2025 | For Retention of Manufacturing Licences | 15000 |
| 217 | 124 | 13/03/2025 | 28/06/2025 | Payment for Grant of Manufacturing Loan License (Payment details of HIMKOSH and Society for SDRS) | 15000 |
| 218 | 257 | 13/03/2025 | 27/03/2025 | SUCCESSFUL | 15000 |
| 219 | 254 | 13/03/2025 | 07/03/2025 | Transation Success of e challan A25C175950, DDO Chief Medical Officer Nahan of amount 15000 | 15000 |
| 220 | 272 | 13/03/2025 | 01/04/2025 | SUCCESSFUL | 15000 |
| 221 | 274 | 13/03/2025 | 01/04/2025 | SUCCESSFUL | 15000 |
| 222 | 258 | 13/03/2025 | 07/03/2025 | A25B302188 & HDFCN52025022886717322 | 30000 |
| 223 | 234 | 15/03/2025 | 24/02/2025 | 15000+130000 IN HIMKOSH & 15000+120000 IN FACILITATION CHARGES | 280000 |
| 224 | 71 | 15/03/2025 | 20/12/2024 | Facilitation Fee also paid with UTR No. SBIN425074800557 | 15000 |
| 225 | 278 | 15/03/2025 | 15/03/2025 | Both Govt. Treasury (HIMKOSH) & Facilitation Charges payment done. Total amount paid 30000. | 15000 |
| 226 | 276 | 16/03/2025 | 01/04/2025 | Sucessful | 15000 |
| 227 | 279 | 19/03/2025 | 19/03/2025 | Depsoited through e-challan no. A25C242958 dated 19.03.2025 and facilitation fees Rs 15000 through UTR reference no. AXSK250780003842 dated 19.03.2025 | 15000 |
| 228 | 279 | 19/03/2025 | 19/03/2025 | Facilitation fees deposited in account of SSDRS though UTR refrence no. AXSK250780003842 dated 19.03.2025 | 15000 |
| 229 | 199 | 19/03/2025 | 19/03/2025 | Drug manufacturer fee for retention | 15000 |
| 230 | 280 | 19/03/2025 | 19/03/2025 | Deposited through E-challan no. A24I125850 dated 04.09.2024 and Facilitation fees vide NEFT Reference no. N5202531721605755 dated 17.03.2025 | 15000 |
| 231 | 280 | 19/03/2025 | 19/03/2025 | deposited SSDSR (Facitation) fees Rs 15000 vide number N52025031721605755 dated 17.03.2025 for retention of Manufacturing licenses | 15000 |
| 232 | 227 | 19/03/2025 | 19/03/2025 | Paid By Tenderer 43265 (Cyno Pharmaceuticals) via Dept Ref No SMNB1098 to Treasury SOL05, DDO 019, HEAD 0210-01-107-01 | 17400 |
| 233 | 227 | 19/03/2025 | 19/03/2025 | Paid By Tenderer 43265 (Cyno Pharmaceuticals) via Dept Ref No SMNB1098 to Treasury SOL05, DDO 019, HEAD 0210-01-107-01 | 17400 |
| 234 | 275 | 20/03/2025 | 20/03/2025 | (i) E-Challan Rs. 7500/- Retention (ii) Callahan for Society for straightening Rs 7500/- Total 15000/- | 7500 |
| 235 | 277 | 20/03/2025 | 20/03/2025 | NEFT O/w-YESBN12025030605819465-punb0413100-Society for strengthening of drugs-2503061113120001 | 7500 |
| 236 | 181 | 21/03/2025 | 28/02/2025 | Fee for Retention of Loan License of Glenmark Pharma c/o Metrocraft Baddi HP | 15000 |
| 237 | 283 | 21/03/2025 | 21/03/2025 | Fees For Retention of Drugs Manufacturing License No. MNB/05/105 & MB/05/106 | 15000 |
| 238 | 286 | 21/03/2025 | 24/04/2025 | State Drugs Controller Baddi | 15000 |
| 239 | 199 | 22/03/2025 | 19/03/2025 | Drug manufacturer fee for retention | 15000 |
| 240 | 288 | 22/03/2025 | 05/04/2025 | Payment for Loan License FDC Limited C/o Prosperity Drugs Pvt. Ltd. | 7500 |
| 241 | 288 | 22/03/2025 | 05/04/2025 | Society for strengthening of Drugs Regulatory System | 7500 |
| 242 | 259 | 22/03/2025 | 05/06/2025 | Fee for grant of loan license | 15000 |
| 243 | 270 | 22/03/2025 | 25/06/2025 | Fee for grant of loan license | 15000 |
| 244 | 87 | 22/03/2025 | 22/03/2025 | FEE TO RETENTION OF DRUG MFG LIC , FEE TO CMO NAHAN | 15000 |
| 245 | 289 | 22/03/2025 | 22/03/2025 | Retention fee for the Drug License | 7500 |
| 246 | 87 | 22/03/2025 | 22/03/2025 | FEE FOR PRODUCTS RETENTION | 150000 |
| 247 | 264 | 24/03/2025 | 02/04/2025 | LICENSE FEES | 30000 |
| 248 | 250 | 24/03/2025 | 24/03/2025 | A25C153507 AND CPAEWXCBW1 AND HDFCN52025030806226731 | 30000 |
| 249 | 250 | 24/03/2025 | 24/03/2025 | A25C153507 AND HDFCN52025030806226731 | 30000 |
| 250 | 87 | 25/03/2025 | 22/03/2025 | FEE FOR PRODUCTS RETENTION | 60000 |
| 251 | 292 | 25/03/2025 | 25/03/2025 | Deposited through e challan no. A25B278863 dated 24.02.2025 | 7500 |
| 252 | 292 | 25/03/2025 | 25/03/2025 | Facilitation fees vide NEFT Reference no. S7261290 dated 24.02.2025 | 7500 |
| 253 | 295 | 25/03/2025 | 25/03/2025 | Retention of Drugs Manufacturing License (Payment details of HIMKOSH and Society for SDRS) | 15000 |
| 254 | 294 | 25/03/2025 | 25/03/2025 | Completed Succesfully | 630000 |
| 255 | 290 | 27/03/2025 | 17/04/2025 | Voizmed Pharma Pvt.Ltd Care of Greef Formulations | 30000 |
| 256 | 291 | 27/03/2025 | 27/03/2025 | Rs. 33,000 paid online in HIMKOSH and Rs. 33,000 NEFT done PNB account of Society for Strengthening of Drug Regulatory System | 66000 |
| 257 | 296 | 28/03/2025 | 09/04/2025 | fees for Drugs manufacturing Loan license | 7500 |
| 258 | 281 | 28/03/2025 | 23/04/2025 | PAYMENT DONE | 15000 |
| 259 | 226 | 31/03/2025 | 22/04/2025 | Rs.15000 | 15000 |
| 260 | 13 | 31/03/2025 | 31/03/2025 | Fees for retention of drugs mfg License on form 28 under Lic MB/09/803 & Extra fees of Rs.7500/- is deposit in himkosh. | 7500 |
| 261 | 297 | 01/04/2025 | 01/04/2025 | Retention Fees of Loan License Cipla Co Immacule Lifesciences Pvt. Ltd. | 7800 |
| 262 | 119 | 01/04/2025 | 27/02/2025 | Both HIMKOSH and Society charges done and receipt and bank statement attached | 15000 |
| 263 | 289 | 01/04/2025 | 22/03/2025 | Retention fee for the Drug License | 7500 |
| 264 | 119 | 01/04/2025 | 27/02/2025 | Both Payment done and attached | 15000 |
| 265 | 13 | 02/04/2025 | 31/03/2025 | Fees for retention of drugs mfg License on form 28 under Lic MB/09/803 & Extra fees of Rs.7500/- is deposit in himkosh | 7500 |
| 266 | 268 | 02/04/2025 | 06/05/2025 | Fifteen Thousand | 30000 |
| 267 | 268 | 02/04/2025 | 06/05/2025 | Thirty Thousand | 30000 |
| 268 | 307 | 02/04/2025 | 24/04/2025 | E-CHALLAN, NEFT | 30000 |
| 269 | 311 | 03/04/2025 | 03/04/2025 | Grant/Retention of Drug Manufacturing License (C, C(1) & X) | 7500 |
| 270 | 313 | 03/04/2025 | 03/04/2025 | LVP | 7500 |
| 271 | 314 | 03/04/2025 | 03/04/2025 | Grant/Retention of Testing Lab | 7500 |
| 272 | 315 | 03/04/2025 | 03/04/2025 | FEES DEPOSITED 15000/- VIDE HIMGRN NUMBER A25D140585 & FOR SDRS RS 15000/- BANK CASH TXN SR NO M1080171/59 FOR THE RETENTION OF DRUG MANUFACTURING LICENSE | 15000 |
| 273 | 316 | 04/04/2025 | 05/04/2025 | Payment for Grant of Manufacturing Loan License (Payment details of HIMKOSH and Society for SDRS) | 15000 |
| 274 | 317 | 05/04/2025 | 24/04/2025 | Sucessful | 15000 |
| 275 | 319 | 05/04/2025 | 04/04/2025 | FEES FOR RETENTION OF DRUGS MANUFACTURING LICENSE WITH LATE FEES | 15000 |
| 276 | 320 | 05/04/2025 | 17/05/2025 | Online and cash payment (Rs 7500 gov. of himachal, Rs 7500 Society for strengthening of drug) | 15000 |
| 277 | 318 | 05/04/2025 | 28/04/2025 | FEES FOR GRANT OF DRUGS MANUFACTURING LOAN LICENSE | 15000 |
| 278 | 299 | 05/04/2025 | 03/05/2025 | Fee for Grant of Loan License | 15000 |
| 279 | 300 | 06/04/2025 | 06/04/2025 | PAYMENT FOR RETENTION OF DRUG MANUFACTURING LICENSE | 30000 |
| 280 | 321 | 06/04/2025 | 06/04/2025 | HIMKOSH: A25C271750 & A25C271975 (15000+170000) AND SOCIETY ACCOUNT : 5197663402 & 5197663543 (15000+170000) | 370000 |
| 281 | 323 | 07/04/2025 | 31/05/2025 | Sucessful | 15000 |
| 282 | 322 | 07/04/2025 | 24/04/2025 | SUCCESSFUL | 15000 |
| 283 | 324 | 07/04/2025 | 07/04/2025 | Retention of Manufacturing License (Payment details of HIMKOSH and Society for SDRS) | 7650 |
| 284 | 293 | 08/04/2025 | 06/06/2025 | FEE FOR GRANT OF LOAN ON FORM-25A and Form-28A (e-Challan Rs. 15000 + SSDRS Rs.15000) | 15000 |
| 285 | 308 | 08/04/2025 | 16/04/2025 | LOAN LICENCE V PROTECT Life Science | 7500 |
| 286 | 310 | 08/04/2025 | 16/04/2025 | LOAN LICENCE DHIGO PHARMA PRIVATE LIMNITED | 7500 |
| 287 | 305 | 08/04/2025 | 20/05/2025 | Fees for Grant of Manufacturing Loan License on form 28A(Payment details of HIMKOSH and Society for SDRS) | 7500 |
| 288 | 326 | 08/04/2025 | 08/04/2025 | License Renewal Fees | 15000 |
| 289 | 306 | 09/04/2025 | 16/04/2025 | Fees for Grant of Drugs Manufacturing loan Licence | 15000 |
| 290 | 226 | 11/04/2025 | 22/04/2025 | Himkosh & Society Fee | 30000 |
| 291 | 327 | 11/04/2025 | 16/04/2025 | Fee for Loan License | 7500 |
| 292 | 331 | 13/04/2025 | 28/04/2025 | FEES FOR GRANT OF LOAN LICENSE | 15000 |
| 293 | 336 | 14/04/2025 | 24/04/2025 | A25C347257,CPAEZAAVE1 & KKBKH25088636675 | 30000 |
| 294 | 363 | 15/04/2025 | 06/05/2025 | DRUG MANUFACTURER -Loan License Retention Fees of Cipla Ltd. Co Ms. Unix Biotech with lic no. L/04/79 (Form 25A) & L/20/2332/MB (Form 28A) | 15300 |
| 295 | 303 | 16/04/2025 | 19/05/2025 | SOCIETY FEES FOR GRANT OF MANUFACTURING LOAN LICENCE | 15000 |
| 296 | 337 | 17/04/2025 | 17/04/2025 | RETENTION MANUFACTURING LICENCE | 15000 |
| 297 | 338 | 17/04/2025 | 17/04/2025 | Retention fee for the Drug Loan License | 15900 |
| 298 | 339 | 17/04/2025 | 23/04/2025 | Fees deposited with application for Lupin Loan Lince at Smilax Pharmachem 33-34, EPIP-II, Thana , Baddi | 30000 |
| 299 | 341 | 18/04/2025 | 18/04/2025 | A25D244546 & M1124928 | 30000 |
| 300 | 226 | 22/04/2025 | 22/04/2025 | fees for grant of loan license | 30000 |
| 301 | 345 | 23/04/2025 | 06/05/2025 | 15000 Payment for Society And 15000 payment for Himkosh (Total Amount Pay 30000 ) | 30000 |
| 302 | 347 | 23/04/2025 | 02/05/2025 | ORISON PHARMACEUTICALS C/O DIGITAL VISION At 176, MAUZA OGLI NAHAN ROAD KALA AMB DISTT. SIRMOUR H.P. 173030 FOR MANUFACTURING LOAN LICENSE FEE | 15000 |
| 303 | 350 | 24/04/2025 | 06/05/2025 | 15000 Payment for Society And 15000 payment for Himkosh (Total Amount Pay 30000 ) | 30000 |
| 304 | 351 | 24/04/2025 | 06/05/2025 | 15000 Payment for Society And 15000 payment for Himkosh (Total Amount Pay 30000 ) | 30000 |
| 305 | 352 | 25/04/2025 | 02/05/2025 | VIGOUR HEALTH CARE PVT. LTD. C/O DIGITAL VISION At 176, MAUZA OGLI NAHAN ROAD KALA AMB DISTT. SIRMOUR H.P. 173030 FOR MANUFACTURING LOAN LICENSE FEE | 15000 |
| 306 | 353 | 25/04/2025 | 06/05/2025 | LOAN LICENSE FEE FOR ACULIFE HEALTH CARE PRIVATE LIMITED C/O VELLINTON HEALTHCARE | 15000 |
| 307 | 348 | 25/04/2025 | 14/05/2025 | Fees for Grant of Manufacturing Loan License on form 28A(Payment details of HIMKOSH and Society for SDRS) | 7500 |
| 308 | 355 | 26/04/2025 | 26/04/2025 | Net banking | 15000 |
| 309 | 355 | 26/04/2025 | 26/04/2025 | Both of challan himkos e-challan 15000 and society for strengthening 15000 | 15002 |
| 310 | 357 | 29/04/2025 | 29/04/2025 | Fee for Retention of Drugs Mfg. License of Glenmark Pharmaceuticals Ltd. (Unit III), Village Kishanpura, Baddi-Nalagarh Road, Tehsil Baddi, Disst. Solan, H.P. 173 205 | 7500 |
| 311 | 358 | 29/04/2025 | 29/04/2025 | FEE FOR RENEWAL OF LOAN LICENSE. Separate facilitation fee amount Rs 15000/- to SSDRS account at PNB Baddi on 15.11.2024 | 15000 |
| 312 | 358 | 29/04/2025 | 29/04/2025 | FEE FOR PRODUCT APPROVAL FEE | 15000 |
| 313 | 346 | 30/04/2025 | 29/04/2025 | FEES FOR RETENTION OF DRUGS MANUFACTURING LICENCE; LATE FEES FOR RETENTION OF DRUGS MANUFACTURING LICENCE; SOCIETY FEES FOR RETENTION OF DRUGS MANUFACTURING LICENCE | 30300 |
| 314 | 126 | 01/05/2025 | 09/06/2025 | Sucessful | 15000 |
| 315 | 362 | 01/05/2025 | 09/05/2025 | NET BANKING | 15000 |
| 316 | 366 | 05/05/2025 | 04/06/2025 | Both of challan himkos e-challan 15000 and society for strengthening 15000 | 15000 |
| 317 | 338 | 06/05/2025 | 17/04/2025 | Retention fee for the Drug Loan License | 15900 |
| 318 | 338 | 06/05/2025 | 17/04/2025 | Retention fee for the Drug Loan License | 15900 |
| 319 | 355 | 07/05/2025 | 26/04/2025 | Both of challan himkos e-challan 15000 and society for strengthening 15000 | 14998 |
| 320 | 369 | 08/05/2025 | 08/05/2025 | FEE FOR RETENTION OF DRUGS MANUFACTURING LICENSE | 15000 |
| 321 | 368 | 08/05/2025 | 04/06/2025 | A25E140801 | 15000 |
| 322 | 373 | 10/05/2025 | 10/05/2025 | FEE FOR RETENTION LOAN LICENSE | 15000 |
| 323 | 376 | 13/05/2025 | 10/06/2025 | Sucessful | 15000 |
| 324 | 373 | 13/05/2025 | 10/05/2025 | FEE FOR RENTENTION LOAN LICENSE | 15000 |
| 325 | 372 | 13/05/2025 | 28/06/2025 | PAYMENT ONLINE SUBMITTED ON DATED 06.05.2025 WITH HIMGRN NO: A25E150765 | 15000 |
| 326 | 372 | 14/05/2025 | 28/06/2025 | Fees for Grant of Manufacturing Loan License on form 25A & 28A (Payment details of HIMKOSH and Society for SDRS) | 15000 |
| 327 | 383 | 14/05/2025 | 14/05/2025 | 564564 | 3000 |
| 328 | 356 | 16/05/2025 | 03/06/2025 | chief medical Officer,Nahan | 15000 |
| 329 | 385 | 17/05/2025 | 19/06/2025 | Both of challan himkos e-challan 15000 and society for strengthening 15000 | 15000 |
| 330 | 374 | 18/05/2025 | 27/06/2025 | Sucessful | 15000 |
| 331 | 382 | 19/05/2025 | 03/07/2025 | 7500rs also deposit in Society A/c R.no. S58696212, dt. 15/05/2025 | 7500 |
| 332 | 390 | 19/05/2025 | 16/06/2025 | dsd11211@#@@@1212~`1`11211@@@ | 212122122 |
| 333 | 390 | 20/05/2025 | 16/06/2025 | Test payment details | 232456 |
| 334 | 390 | 20/05/2025 | 16/06/2025 | TEst payment Details | 4000 |
| 335 | 391 | 20/05/2025 | 20/05/2025 | Rentention Fee for Drugs Licesne | 30000 |
| 336 | 390 | 20/05/2025 | 16/06/2025 | redf | 45865 |
| 337 | 390 | 20/05/2025 | 16/06/2025 | Rwwe | 1212212 |
| 338 | 390 | 20/05/2025 | 16/06/2025 | fsdf | 34 |
| 339 | 236 | 20/05/2025 | 10/03/2025 | License Renewal Fees | 15600 |
| 340 | 397 | 21/05/2025 | 21/05/2025 | A25E201775 DT 13.05.2025 & 3378 & M905360 07.05.2025 | 15000 |
| 341 | 392 | 21/05/2025 | 06/06/2025 | Grant of Manufacturing Loan License (Payment details of HIMKOSH and Society for SDRS) | 7500 |
| 342 | 399 | 22/05/2025 | 22/05/2025 | Fee for retention of Drugs manufacturing Licenses Nos MB/20/1094 and also fee for society enclosed with challan | 7500 |
| 343 | 402 | 23/05/2025 | 23/06/2025 | FEE FOR THE GRANT OF DRUGS MANUFACTURING LOAN LICENSE | 7500 |
| 344 | 387 | 23/05/2025 | 03/06/2025 | 15000 | 15000 |
| 345 | 387 | 23/05/2025 | 03/06/2025 | A25E217159 | 15000 |
| 346 | 406 | 24/05/2025 | 31/05/2025 | A25E293091,CPAFDWSWW6, Society payment done Ref no. ST24INS1748081579 | 15000 |
| 347 | 403 | 24/05/2025 | 06/06/2025 | Transation Success of e challan A23H218850, DDO Chief Medical Officer Nahan of amount 7500 | 7500 |
| 348 | 407 | 24/05/2025 | 06/06/2025 | Transation Success of e challan A25E293363, DDO Chief Medical Officer Nahan of amount 7500 | 7500 |
| 349 | 409 | 25/05/2025 | 06/06/2025 | Transation Success of e challan A25E293442, DDO Chief Medical Officer Nahan of amount 7500 | 7500 |
| 350 | 412 | 27/05/2025 | 19/06/2025 | Net Banking | 15000 |
| 351 | 411 | 28/05/2025 | 27/05/2025 | challan Society 7500 on dated 14/05/2025 receipt no 57090848 | 7500 |
| 352 | 413 | 28/05/2025 | 27/05/2025 | A25E317052 | 7500 |
| 353 | 376 | 28/05/2025 | 10/06/2025 | SUCCESSFULLY | 15000 |
| 354 | 415 | 29/05/2025 | 07/07/2025 | FEE FOR GRANT OF LOAN LICENSE | 15000 |
| 355 | 417 | 29/05/2025 | 29/05/2025 | Scoety challan no 57085743 Dated 14/05/2025 paid 7500 | 7500 |
| 356 | 408 | 29/05/2025 | 26/06/2025 | Sucessful | 15000 |
| 357 | 416 | 30/05/2025 | 13/06/2025 | Payment for Grant of Manufacturing Loan License (Payment details of HIMKOSH and Society for SSDRS) | 15000 |
| 358 | 386 | 30/05/2025 | 19/06/2025 | FEE FOR GRANT OF DRUGS MANUFACTURING LOAN LICENSE | 15000 |
| 359 | 396 | 30/05/2025 | 28/06/2025 | Payment for Grant of Manufacturing Loan License (Payment details of HIMKOSH and Society for SSDRS) | 7500 |
| 360 | 401 | 02/06/2025 | 23/05/2025 | RETENTION OF LVP LICENSE NO LVP/09/05 | 7500 |
| 361 | 410 | 03/06/2025 | 28/06/2025 | A25F109545 | 15000 |
| 362 | 400 | 04/06/2025 | 04/06/2025 | Fee for retention of Drug Manufacturing Loan License and also Society Fee payment receipt enclosed with the challan | 7500 |
| 363 | 418 | 05/06/2025 | 27/06/2025 | FEES DEPOSITED FOR MANUFACTURING LICENCE | 15000 |
| 364 | 419 | 05/06/2025 | 05/06/2025 | Renewal of Manufacturing Loan License ( 15000 in HIMKOSH and 15000 in Society Account ) | 15000 |
| 365 | 365 | 06/06/2025 | 06/06/2025 | MANUFACTURING LICENCE RETENTION | 15000 |
| 366 | 414 | 06/06/2025 | 06/06/2025 | Retention of Loan License | 15000 |
| 367 | 54 | 09/06/2025 | 22/11/2024 | RENEWAL FEES MNB 1096 | 7500 |
| 368 | 54 | 09/06/2025 | 22/11/2024 | FEE FOR RETAINED PRODUCTS | 35000 |
| 369 | 420 | 09/06/2025 | 06/06/2025 | A25F146925 & M206317 (3731) | 32000 |
| 370 | 426 | 12/06/2025 | 23/06/2025 | A25E217159 | 15000 |
| 371 | 333 | 13/06/2025 | 22/04/2025 | Payment for loan license renewal of Alisha & Isha Pharmaceuticals | 15600 |
| 372 | 428 | 13/06/2025 | 23/06/2025 | 7500 | 7500 |
| 373 | 428 | 13/06/2025 | 23/06/2025 | LONE LICENCE FEE | 7500 |
| 374 | 432 | 16/06/2025 | 16/06/2025 | Retention Loan | 3000 |
| 375 | 423 | 17/06/2025 | 02/07/2025 | Deposited through e challan no. A25F133004 | 15000 |
| 376 | 423 | 17/06/2025 | 02/07/2025 | Deposited fees e-challan no. A25F133004 dated 05.06.2025 and facilitation fees of Rs 15000/- vide receipt no. M1070445 dated 04.06.2025 | 15000 |
| 377 | 435 | 17/06/2025 | 17/06/2025 | From 19 Retail drugs license | 1212121 |
| 378 | 435 | 17/06/2025 | 17/06/2025 | sdsa | 111 |
| 379 | 435 | 18/06/2025 | 17/06/2025 | Test123@ | 123 |
| 380 | 430 | 18/06/2025 | 18/06/2025 | Fee for Retention of Manufacturing License | 15000 |
| 381 | 441 | 21/06/2025 | 07/07/2025 | ASHWIN VORA | 15000 |
| 382 | 441 | 21/06/2025 | 07/07/2025 | ASHWIN VORA | 15000 |
| 383 | 441 | 21/06/2025 | 07/07/2025 | Fee for grant of Loan License | 15000 |
| 384 | 443 | 23/06/2025 | 27/06/2025 | Sucessful | 15000 |
| 385 | 444 | 23/06/2025 | 24/06/2025 | sdgsahdja | 23213213 |
| 386 | 444 | 23/06/2025 | 24/06/2025 | tetre | 233 |
| 387 | 444 | 24/06/2025 | 24/06/2025 | erwer | 232 |
| 388 | 450 | 26/06/2025 | 26/06/2025 | Retention of Loan License at Alkem L | 15000 |
| 389 | 448 | 26/06/2025 | 07/07/2025 | HIMKOSH & PNB FEES | 30000 |
| 390 | 379 | 27/06/2025 | 13/05/2025 | Fees for Retention of Drugs Manufacturing License | 15000 |
| 391 | 444 | 30/06/2025 | 24/06/2025 | dsdfdfds | 34343 |
| 392 | 458 | 04/07/2025 | 04/07/2025 | Fees paid for the Retention of GSK manufacturing Loan License (in Form 28A) No. L/20/2406/MB and the product permission fees for 06 products 7500+1800=9300 We also have deposited the mentioned facilitation charges in saving account =9300 | 9300 |
| 393 | 459 | 06/07/2025 | 06/07/2025 | FEE FOR MANUFACTURING LICENCE RETENTION | 15000 |
27 day(s) 18 hr(s)
21 day(s) 0 hr(s)
0 day(s) 0 hr(s)
199 day(s) 1 hr(s)
Bharat
© ,ONLINE DRUGS LICENSING SYSTEM. All Rights Reserved. Number of visitors: 0 Terms & Conditions